Depression is a common form of mood disorder affecting more than 264 million people worldwide [1]. Prevalence of depression among elderly persons in India is 21.9% as per study report [2]. In the most recent surveys, MDD has the high lifetime prevalence of any psychiatric disorder in different countries [3].
Although the first antidepressant drugs, the Monoamine Oxidise Inhibitors (MAOIs) and Tri Cyclic Antidepressants (TCAs) are still in use, newer compounds like Selective Serotonin Reuptake Inhibitors (SSRIs) are preferred for better ADR profile. The six currently available SSRIs namely fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, escitalopram vary in their pharmacokinetic properties, tolerability and adverse profile [4]. The much promoted advantages are towards relative safety and better acceptability, little effects on sedation, non-interference with cognitive and psychomotor function, and absence of anticholinergic adverse effects [4]. However, the validity of this claim has been challenged by findings from different studies showing treatment discontinuation due to their ADRs mainly fluoxetine and sertraline [5]. Patient awareness about the potential of drugs to cause adverse reactions has remained an important strategy towards ensuring medicine safety [6]. Among the ADRs, hyponatremia is an under-recognised but potentially lethal complication in patients treated with SSRIs especially in elderly [7].
There is relative paucity of published literature on the safety and tolerability of SSRIs antidepressants, in Indian patients. As there are only few published studies regarding effects of SSRIs on serum sodium level in human [8,9], The present study was done to assess the safety, tolerability and awareness through prescription event monitoring with special emphasis on hyponatremia. Information on animal model is also lacking as per literature review [10-12]. An animal “system” which has predictive validity to human responses or physiological processes will be a good model. So it was decided to carry out the study to find out association of hyponatremia with SSRI in both human and animal model. Exploring asymptomatic hyponatremia associated with SSRI especially in elderly patients will be helpful in clinical decision making process. Hence, primary objective of this study was to determine SSRIs induced hyponatremia in human and its correlation with age. To investigate the occurrence of hyponatremia relative to the time of initiating treatment with SSRI. To find out relationship between human and animal model with special reference to SSRI induced hyponatremia were the secondary objectives.
Materials and Methods
Present study has two parts, one is clinical part (prospective cohort study) and another is experimental part. Study was carried out over a period of March 2012 to October 2013 for animal experimentation. In Clinical part, consecutive sampling method was used. Using G*Power 3.1.9.4, sample size calculation was done to detect medium effect size of 0.25 with 80% power for between group comparison, alpha was set at 0.05. Between groups comparison (one-way ANOVA) required 200 patients and was large in comparison to other method of analysis, 10% drop out rate included. For calculation of sample size in this animal experiments, we used “resource equation” method which is based on law of diminishing return. As reports of previous animal studies are lacking, we preferred this method. A value “E” is measured, which is the degree of freedom of analysis of variance (ANOVA). The value of E between 10 and 20 should be considered as an adequate and is given by the formula E=Total number of animals-Total number of groups. E in this study is 25. We had 5 groups and sample size came to (30-5), assuming 20% loss (5), final sample size became 30 [13-15].
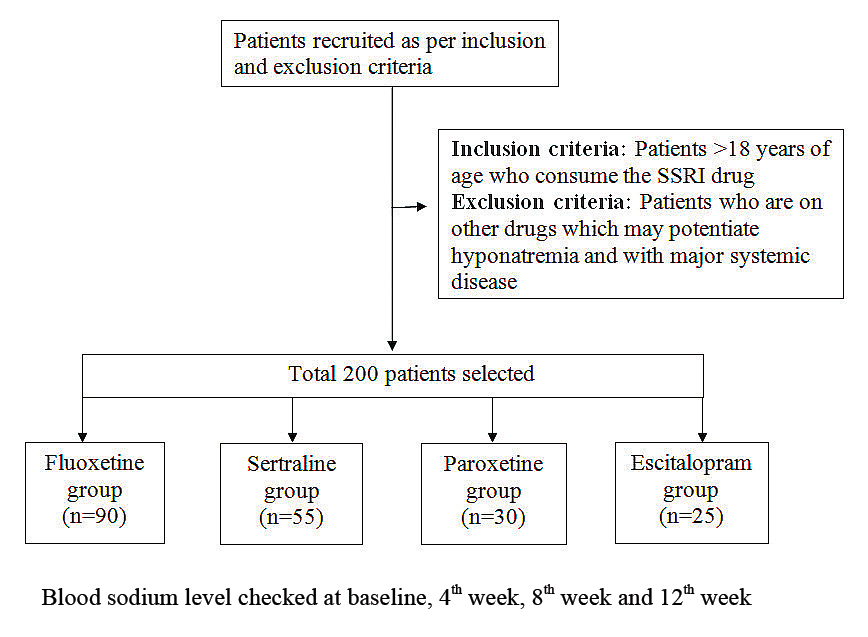
Inclusion criteria: Patients of either sex, aged above 18 years, attending the Out-Patient’ Department (OPD) of the Department of Psychiatry and diagnosed as MDD by DSM 5 criteria [16] were screened and recruited (newly diagnosed cases) with the help of psychiatrist in the study.
Exclusion criteria: Patients with co-existing chronic illness such as diabetes, hypertension, cardiac, hepatic and renal ailments, those patients who were on any other drug which may potentiate hyponatremia and with major systemic disease were excluded from the study.
Protocol designing and animal experiment was done in Pharmacology Department and biochemical tests were done in Biochemistry Department. Subject recruitment was started only after Ethics Committee Approval in writing by the concerned authority. They were followed-up for baseline and another three visits (4th weeks, at 8th weeks and at 12th weeks) at scheduled intervals. Patients with moderate to severe symptoms along with other clinical profiles were prescribed either of the four commonly used SSRIs-fluoxetine, sertraline. paroxetine, escitalopram in oral routes as per National Institute for Health and Care Excellence (NICE) guide line [17]. The study conformed to the Declaration of Helsinki [18] (as revised in 2013) and Indian Council of Medical Research (ICMR) ethical guidelines for clinical research [19].
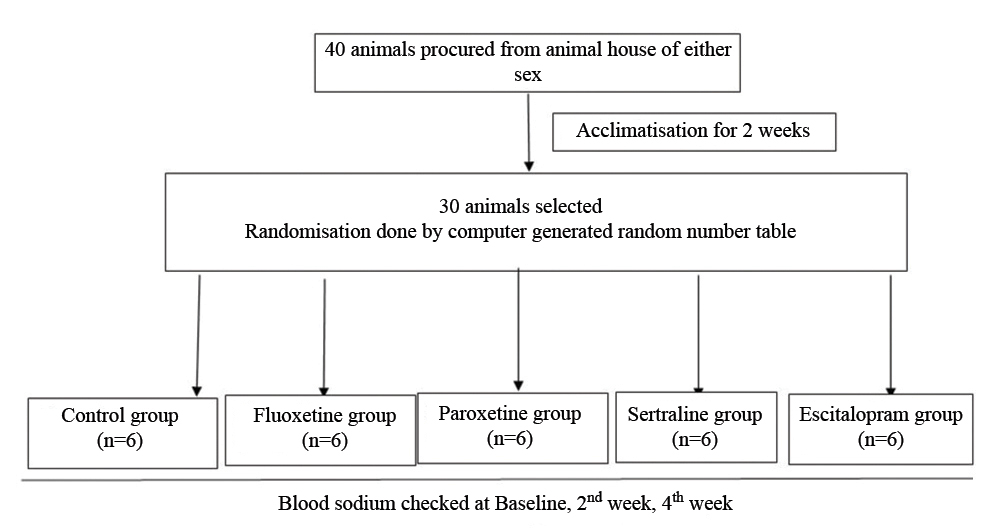
All experiments were conducted with due care in accordance to Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and Good Laboratory Practice rules and guidelines [20,21]. The experimental part included Albino rats of both the sexes with average body weight (100-200 grams) were brought from the central animal house. Total 30 housed and allowed an acclimatisation period of 2 weeks [20] before the beginning of the experiment. The animals were maintained in groups of both sexes, three per cage under controlled temperature (22°C) and humidity (50-60 %) condition. The rats were randomised into four test group (n=6) and one control group (n=6). The initial baseline or pre-treatment blood levels of sodium were checked. The test group of rats of both the sexes was treated with four SSRIs (fluoxetine, sertraline, paroxetine and escitalopram) with respective dose of 10 mg/kg body weight [22], 15 mg/kg body weight [23], 5 mg/kg/body weight [24], 3 mg/kg/body weight [25] respectively per oral. Fluoxetine hydrochloride tablets (20 mg) and Sertraline hydrochloride (50 mg) were procured from the hospital pharmacy. Paroxetine (10 mg), escitalopram tab (10 mg) was taken from outside pharmacy store. Each tablet was dissolved in 5 mL of distilled water and accordingly the volume of the drug solution to be administered was calculated and administered for a period of 6 weeks (sub-chronic administration). Controls were given the same volume of distilled water as vehicle. The treatment continued (1 dose/day) for four weeks. The blood samples were collected for sodium level monitoring at pre-treatment baseline and post-treatment 2nd week and 4th week follow-up. These effects were compared with vehicle control group. For clinical part, pre-validated Questionnaire for assessing patients’ perception about ADR potentials of SSRIs by structured interview has been used. ADR reporting form was used for ADR Monitoring and causality assessment one by WHO’s causality assessment scale [26]. The outcome hyponatremia was defined as plasma sodium below 135 mmol/L [27]. [Table/Fig-1,2] provides a flowchart of study related activities.
Study flow chart (Clinical part).
Study flow chart (Animal study).
Statistical Analysis
After collection of data, frequency distribution tables were prepared. Numerical value along with percentages were described and categorical data were coded accordingly and then were analysed using SPSS Version 17. For clinical part of the study, distribution of age was tested by one-way ANOVA, gender and socio-economic strata by chi-square test. The changes of blood sodium level in comparison with pre-treatment baseline values and the subsequent follow-ups were analysed, between group comparison by one-way ANOVA followed by post hoc and within groups by repeated measures ANOVA followed by post-hoc. Chi-square test was used to detect any difference in symptoms due to hyponatremia in different groups. Results of adverse events in different drug groups were analysed also with Chi-square test. Association between age and serum sodium level was tested using Pearson product moment correlation (bivariate). In similar way, changes in different parameters were analysed in experimental part and considering non-normality of distribution pattern of data, non-parametric test were carried out. Between group comparison by Kruskal Wallis test and within group comparison by Friedman’s ANOVA were done.
Results
Gender distribution was equal and the patients were in between 40-50 years, indicating preponderance to middle aged to elder age groups. Study groups were comparable at baseline with respect to age and sex [Table/Fig-3].
| Parameters | Fluoxetine (n=90) | Sertraline (n=55) | Paroxetine (n=30) | Escitalopram (n=25) | p-value (between groups) |
|---|
| Age (yrs) RangeMean±SD | 28-7447.97±15.14 | 28 to 7450.75±14.14 | 28 to 7443.73±14.34 | 28 to 7443.72±15.16 | 0.100 |
| Sex |
| Male | 40 (44.5%) | 30 (54.5%) | 15 (50%) | 15 (60%) | 0.177 |
| Female | 50 (55%.5) | 25 (45.5%) | 15 (50%) | 10 (40%) |
| Income (monthly) |
| <Rs. 5000 | 29 (32%) | 7 (12.7%) | 13 (43%) | 13 (52%) | <0.001 |
| Rs. 5000-10,000 | 50 (55.5%) | 46 (83.6%) | 14 (46.6%) | 9 (36%) |
| ≥Rs. 10,000 | 11 (12%) | 2 (3.6%) | 3 (10%) | 3 (12%) |
p-value for age by one-way ANOVA, gender and socio-economic status by chi-square test; p-value <0.05 was considered statistically significant
The changes of blood sodium level in comparison with pre-treatment baseline values and the subsequent follow-ups showed significant results. Blood level of sodium at the baseline between different groups were comparable (p>0.05). In follow-up periods, changes in blood level among four groups were statistically significant (p<0.05) as found in one-way ANOVA. In final follow-up, it is highly significant at 0.003 level though decrease in sodium level started from first follow-up and continued to final follow-up period [Table/Fig-4].
Changes in blood sodium level between different SSRIs.
| Changes in blood sodium level | Baseline | 1st Follow-up (4th week) | 2nd Follow-up (8th week) | Final follow-up (12th week) | p-value (within groups) |
|---|
| Fluoxetine Mean±SD | 139.32±2.35 | 130±3.86* | 129.68±3.42* | 129.61±3.34* | <0.0001 |
| Sertraline Mean±SD | 139.33±2.24 | 128.53±2.75* | 128.58±2.87* | 130.60±3.34* | <0.0001 |
| Paroxetine Mean±SD | 139.73±2.35 | 130.03±3.43* | 130.60±3.34* | 130.60±3.34* | <0.0001 |
| Escitalopram Mean±SD | 139.84±2.27 | 131.24±3.94* | 130.92±3.94* | 130.72±3.44* | <0.0001 |
| p-value (between groups) | 0.666 | 0.003 | 0.012 | 0.003 | |
p-value within groups by repeated measures ANOVA followed by post-hoc.
*Denotes significant changes from baseline for that follow-up (p-value for within groups by one-way ANOVA followed by post-hoc); p-value <0.05 was considered statistically significant
In the present study, it was also seen that most of people were asymptomatic, some developed mild non-specific symptoms like headache, lethargy, drowsiness, anorexia etc., [Table/Fig-5]. Symptoms reported due to hyponatremia were nausea and vomiting, headache, confusion, lethargy/fatigue, restlessness/irritability mainly in fluoxetine and sertraline group. Correlation between Na levels and age of the patient in different follow-up shows negative correlation coefficient (≥0.783) and were statistically significant [Table/Fig-6].
Symptoms due to hyponatremia.
| Symptoms due to hyponatremia | Fluoxetine (n=90) | Sertraline (n=55) | Paroxetine (n=30) | Escitalopram (n=25) | p-value |
|---|
| Present | 10 (11%) | 4 (7%) | 0 (0%) | 2 (8%) | 0.280 |
| Absent | 80 (89%) | 51 (93%) | 30 (100%) | 23 (92%) | |
p-value by Chi-square test
Pearson’s correlation between Na levels and age in different follow-up.
| Fluoxetine | Sertraline | Paroxetine | Escitalopram |
|---|
| Follow-up | Na levels (r) | Na levels (r) | Na levels (r) | Na levels (r) |
| Baseline | 139.32±2.35 (-0.2512) | 139.33±2.24 (-0.08599) | 139.73±2.35 (-0.4426) | 139.84±2.27 (-0.3879) |
| 1st follow-up | 130±3.86 (-0.7448) | 128.53±2.75 (-0.7901) | 130.03±3.43 (-0.7421) | 131.24±3.94 (-0.8124) |
| 2nd follow-up | 129.68±3.42 (-0.793) | 128.58±2.87 (-0.7747) | 130.60±3.34 (-0.8009) | 130.92±3.94 (-0.8126) |
| 3rd follow-up | 129.61±3.34 (-0.7893) | 130.60±3.34 (-0.7934) | 130.60±3.34 (-0.8007) | 130.72±3.44 (-0.8128) |
Treatment emergent clinical adverse events: All the patients who received at least one dose of the treatment medication were analysed. This included all 200 patients divided into four treatment arms. During the 16 weeks of study period, a total of 168 subjects were suspected of having at least one ADR, while two patients receiving sertraline therapy developed hyponatremia associated with seizure, led to discontinuation of the drug immediately and admitted to hospital for conservative management. On causality assessment, 30 of these 168 cases (17.86%) were considered to have insufficient evidence about causality World Health Organisation (WHO)-Uppsala Monitoring Centre (UMC) causality status “unlikely”) and they were excluded from further analysis. From the remaining 136 subjects, 189 ADRs were tabulated. Causality assessment revealed that 72 ADRs (38.09%) belonged to “probable” category, 5 (2.64%) “possible”, 86 (45.50%) Unlikely [26], 10 (5.29%) Unclassified and 16 (8.46%) Unassessable type according to the WHO-UMC scale [26].
No case could be labeled “certain”, as rechallenge was not attempted by the attending psychiatrist, once a drug was withdrawn. Overall, out of 189 treatment-emergent adverse events from 136 subjects: 60 subjects (66.6% of the 90 patients) in the fluoxetine arm and 40 subjects (72.72% of the 55 paients) in the sertraline arm, 20 subjects (66.6% of the 30 patients) and 16 subjects (64% of the 25 patients) reported at least one event. Statistically, all groups all were comparable [Table/Fig-7].
| Awareness regarding adverse events | Fluoxetine (n=90) | Sertraline (n=55) | Paroxetine (n=30) | Escitalopram (n=25) | p-value |
|---|
| Yes | 60 (66.66%) | 40 (72.72%) | 20 (66.66%) | 16 (64%) | 0.800 |
| No | 30 (33.33%) | 15 (27.37%) | 10 (33.33%) | 9 (36%) |
p-value by Chi-square test
Again when looked upon in the experimental set-up, white albino rats showed a gradual decrease in blood sodium level from the very first week after administration of SSRIs (fluoxetine, sertraline, paroxetine and escitalopram), which showed significant difference at post-treatment weeks in comparison to baseline values [Table/Fig-8]. Hence, in both clinical as well as in the experimental part of this study SSRIs (fluoxetine, sertraline, paroxetine and escitalopram) treatment has shown significant hyponatremia, mainly asymptomatic in nature.
Changes in Na between different SSRIs in animal study.
| Changes in Na | Baseline (mEq/L) | 2nd week (mEq/L) | 4th week (mEq/L) | p-value (within groups) |
|---|
| Control group Mean±SD (mEq/L) | 139.32±2.34 | 137.45±2.67 | 138.24±3.12 | 0.0204 |
| Fluoxetine Mean±SD (mEq/L) | 139.32±2.35 | 131.57±3.11* | 130±3.86* | <0.001 |
| Sertraline Mean±SD (mEq/L) | 139.33±2.24 | 131.57±3.11* | 128.53±2.75* | 0.002 |
| Paroxetine Mean±SD (mEq/L) | 139.73±2.35 | 131.90±2.77* | 130.03±3.43* | 0.002 |
| Escitalopram Mean±SD (mEq/L) | 139.84±2.27 | 132.0±2.37* | 131.24±3.94* | <0.001 |
| p-value (between groups) | 0.333 | 0.514 | 0.070 | |
p-value between groups by Kruskal Wallis ANOVA; *Denotes significant changes from baseline for that follow-up (for within group analysis); p-value within groups by Friedman’s ANOVA followed by post-hoc Dunn’s test; Paired t-test for comparison between baseline and final follow-up (within group comparison); p-value <0.05 was considered statistically significant
Discussion
The first part of the present study was carried out as a hospital based study in a tertiary care hospital. It is observed that there is an increasing tendency of prescribing SSRIs, has substantially improved the ease of treating depression. Choice of drug was made jointly between the patient and the health professional. Probably the prescribing pattern was more influenced by the socio-economic status of the patients and availability of the SSRIs in the hospital pharmacy. A multicenter study done in 40 centers also found those factors influence prescribing pattern [28]. Hyponatremia is common form of electrolyte disturbance amongst psychiatric patient [29] and hyponatremia is known to be associated with many different drugs including diuretics, anti-epileptics and psychotropic drugs. In this study, authors defined hyponatremia as blood sodium <135 due to the fact that even mild hyponatremia during recent years have been shown to be of clinical importance [27]. All SSRIs produced hyponatremia in these settings which was statistically significant changes of sodium between SSRI.
Prior evidence for the association between anti-depressants and hyponatremia mainly arises from different studies [27,29,30]. Different studies support that fluoxetine had a higher risk for developing other hyponatremia as compared with other anti-depressant drugs [27,29]. In the WHO database of ADRs, most reports of SSRI-related hyponatremia were due to fluoxetine, paroxetine and sertraline [5]. The Therapeutic Goods Administration (TGA) has reported that SSRIs induced hyponatremia in Australia. Between Januarys 2009-2011, the TGA received 136 reports of drug induced hyponatremia, in which it was seen that SSRIs were implicated in 22% [31]. Although reports of severe SSRI-induced hyponatremia original clinical trials are rare, the incidence remain in 1 in 200 elderly patients per year receiving treatment with fluoxetine or paroxetine [5]. On the other, hand hyponatremia due to SIADH is less common in patients treated with other SSRIs and venlafaxine [32].
In the present study, it is clearly seen that hyponatremia due to fluoxetine, sertraline, paroxetine and escitalopram were strongly correlated with age. It was also seen that most of people were asymptomatic, some developed mild non-specific symptoms like headache, lethargy, drowsiness, anorexia etc., while two patients receiving sertraline therapy developed hyponatremia associated with seizure, led to discontinuation of the drug immediately and admitted to hospital for conservative management. It is interesting to note that mild or moderate hyponatremia can be overlooked in elderly depressed patients because of non-specific symptoms-including anorexia, nausea, fatigue, lethargy, and confusion-are common in this patient population. Elderly patients are particularly at risk for hyponatremia in general, since many are taking diuretics for hypertension control, which some studies have shown to increase the risk of SSRI-associated hyponatremia [33]. Dementia and other co-morbid conditions in elderly patients may mask its onset or may change its severity [34].
In this study, hyponatremia developed a mean of 9 days after SSRIs were started (range 1-14 days), not dose related and did not correlate with plasma concentrations of SSRIs. Hyponatremia associate with antidepressants can occur from within one day to several months after the initiation of drug therapy. Liu MK et al., reported median onset of 13 days [29]. Ayus JC and Moritz ML also found hyponatremia in a time range of 1 to 253 days with a mean time of approximately 3 weeks after initiation of therapy [35]. In a study by Ferguson JM, the median time to onset was 14 days [5]. Escitalopram showed similar variation in onset of hyponatremia after medication [36-38].
Animal part: The animal experimental part showed that there were significant changes in blood sodium level between pretreatment baseline value and post-treatment values. There are only few published studies regarding effects of SSRIs on serum sodium level in animal model and the proposed mechanism [10-12]. Sertraline showed changes in thirst and appetite in sertraline-treated rats in basal conditions and after osmotic/volumic challenges due to inhibitory influence of the serotonergic system on water intake [39]. That might be acting simultaneously with the hyponatremia, higher plasma volume and higher Arginine Vasopressin (AVP) plasma levels observed in sertraline-treated rats. These results supported data from other studies [40-42] in which an association between the syndrome of inappropriate Secretion Of Antidiuretic Hormone (SIADH) and hyponatremia in patients under chronic SSRI treatment was observed. Study by Catalano MC et al., found no change in plasma sodium level with sertraline treatment in humans [43]. Hyponatremia observed after sertraline treatment with increased plasma levels of vasopressin and oxytocin in rats [43]. Additionally, the present data alerted us to be aware of the risk of iatrogenic induction of a severe hyponatremia in patients submitted to chronic treatment with SSRI, particularly when combined with diuretic therapy.
In present study, the authors found out there is significant difference between control and four test groups in comparison to blood sodium level in final follow-up with paired T-test. The drug treated animals revealed significant difference in blood sodium level in comparison to baseline. Most of the patients in this study were from the lower socio-economic strata of the society [Table/Fig-3] who were unable to state and even explain the nature of their adverse events. During the structured interview, it was quite difficult to communicate with the patients. Also, few patients missed their scheduled follow-up for one or two visits, and were interacted as and when they turned up and blood level of sodium was measured accordingly by patients’ recall as far as possible. During maintenance of the rats by resident intruder paradigm, a separate animal room with selected glass panes and uninterrupted electric supply to maintain the reverse light and dark cycle were really very difficult tasks to achieve.
Limitation(s)
In this study, pathogenesis of hyponatremia in patients treated with SSRIs specifically is unknown. Some animal studies have shown that serotoninergic mechanisms are involved in the regulation of antidiuretic hormone secretion; The syndrome of inappropriate antidiuretic hormone secretion has often been mentioned in case reports as the cause of antidepressant-associated hyponatremia. Potent confounding factors in this study were family history of psychiatric illness, history of addiction, concomitant medications.
Conclusion(s)
Present study addresses the most recent evidence on the prevalence of hyponatremia in patients treated for depression without co-morbid severe mental illness, focusing specifically on the relative rates of hyponatremia. It was found that fluoxetine, sertraline, paroxetine and escitalopram are strongly associated with hyponatremia and is common in elderly patients. History of other medications (such as diuretics, drugs acting on CNS) along with SSRI in elderly patients is very important as most of those who developed hyponatremia remained either asymptomatic or reported only mild nausea or fatigue. Failure to detect and manage mild hyponatremia may result in progression to moderate or severe hyponatremia that can lead to seizures, coma, or death. For this reason routine monitoring of sodium concentrations in patients prescribed an SSRI is essential. Author suggests to carry out study to explore underlying mechanisms involved in antidepressant induced hyponatremia.
p-value for age by one-way ANOVA, gender and socio-economic status by chi-square test; p-value <0.05 was considered statistically significant
p-value within groups by repeated measures ANOVA followed by post-hoc.
*Denotes significant changes from baseline for that follow-up (p-value for within groups by one-way ANOVA followed by post-hoc); p-value <0.05 was considered statistically significant
p-value by Chi-square test
p-value by Chi-square test
p-value between groups by Kruskal Wallis ANOVA; *Denotes significant changes from baseline for that follow-up (for within group analysis); p-value within groups by Friedman’s ANOVA followed by post-hoc Dunn’s test; Paired t-test for comparison between baseline and final follow-up (within group comparison); p-value <0.05 was considered statistically significant