Onychomycosis is a primary fungal infection invading the healthy nail plaque or develop in older adults due to peripheral vascular disease, immunologic disorders, and diabetes mellitus [1]. It is though not fatal still is clinically relevant due to its interference with patient’s quality of life, leading to cosmetic impairment, as well as to low esteem and compromising the functional capacity [2,3].
Onychomycosis may be of different clinical spectrums such as Distal Lateral Subungual Onychomycosis (DLSO), Superficial White Onychomycosis (SWO), proximal subungual onychomycosis, endothrix onychomycosis and Total Dystrophic Onychomycosis (TDO) [5]. As the aetiology of onychomycosis varies, the fungi have a variable susceptibility to antifungal drugs and treatment involves long duration antifungal therapy [6]; so empirical treatment for a longer duration may lead to resistance. Laboratory diagnosis including speciation by culture and antifungal susceptibility is essential to establish disease aetiology and help choose the best therapeutic option.
In view of above, this study was designed to aware the clinicians about the prevalence of onychomycosis in Odisha, it’s demographic link, different dermatophytic and non-dermatophytic causes with effective antifungals to guide the clinician for better treatment planning for this disease.
Materials and Methods
Over a period of two years (July 2018-June 2020) for this prospective study, a total of 300 nail samples from patients with nail infection were collected after taking proper consent and was processed in the Department of Microbiology at IMS and SUM Hospital, Bhubaneswar, Odisha, India. Patients using topical or oral antifungal drugs at the time of sample collection or up to 15 days before the day of collection and patients with insufficient specimen were excluded from the study. Nail material was taken from clinically abnormal nails or from the first right toenail if all nails appeared normal. Nails were cleaned with alcohol and nail clippings were collected on a sterile black filter paper or cardboard folder. The sample was divided in two portions: one part for fungal culture and another part for microscopy. The wet mount for microscopic examination was prepared using 20% KOH and examined after overnight incubation. In scanty sample, a drop of parker blue black ink was used to reduce false negative rates.
Study Procedure
Culture was done in duplicate in SDA tubes; one with actidione (cycloheximide) and another without it and both were incubated at 25°C for four weeks in BOD incubator. Samples were considered negative if no growth was seen after four weeks of incubation. Positive cultures growing dermatophytes and moulds [Table/Fig-1] were processed further for identification and speciation by tests like Lactophenol Cotton Blue (LPCB) staining, slide culture and urease production. Gupta AK et al., inoculums counting (Walshe and English criteria) method was followed for diagnosis of non-dermatophytic filamentous fungi [7]. Isolation of dermatophyte was considered as a pathogen irrespective of direct microscopy result.
SDA tubes showing growth of Candida, Aspergillus and Trichophyton spp.
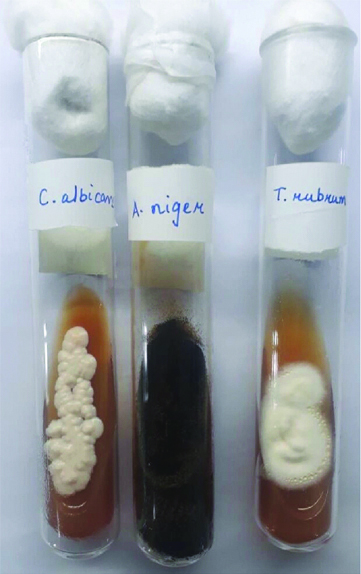
Culture tubes growing yeasts [Table/Fig-1] were further subjected to speciation by germ tube test, culture on CHROM agar (HiChrome Candida differential agar- Himedia) [Table/Fig-2] and on cornmeal agar (Dalmau plate culture).
Different Candida spp. Colonies on CHROM agar plate.
Green coloured colonies- C. albicans; white to pale pink coloured colonies- Candida parapsilosis; Metallic Blue coloured colonies- Candida tropicalis
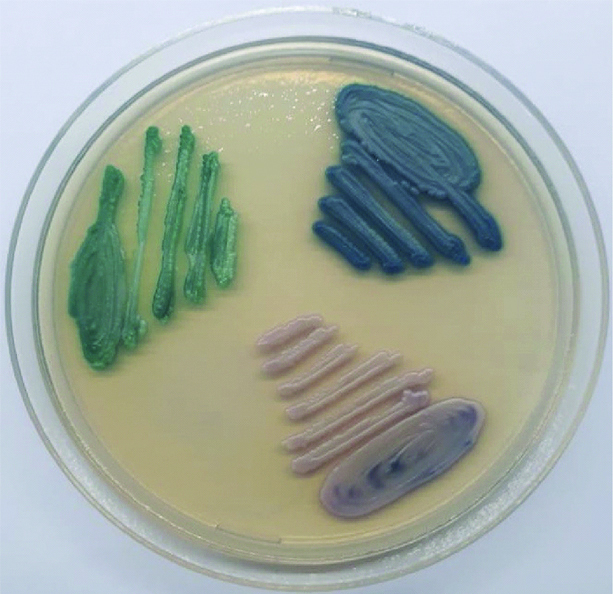
In-vitro antifungal susceptibility testing was performed against Candida species using disc diffusion method on Muller Hinton agar with 2% glucose and methylene blue for fluconazole (10 μg), itraconazole (10 μg) and amphotericin B (20 μg) as per CLSI guidelines [8].
Statistical Analysis
Statistical Analysis was done as per Chi-square test and data was analysed by Microsoft Excel software and p-value of <0.05 was considered significant.
Results
In the present study, out of total of 300 clinically suspected onychomycosis cases, most of them were in the age group of 31-40 years, 41-50 years and 21-30 years with involvement of 99/300 (33%), 69/300 (23%) and 52/300 (17.3%) patients, respectively [Table/Fig-3]. In any of these patients, involvement of both toenails and fingernails was not found. Most of the fingernail’s involvement (144/201, 71.7%) was found in females.
Demographic details of onychomycosis patients.
| Age group (Years) | Occupation | Site of Nail Involvement | Total | Total |
|---|
| Housewife | Farmer | Student | Business | Service | Others | Finger | Toe | (%) |
|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M+F |
|---|
| <20 | 0 | 0 | 0 | 0 | 10 | 12 | 4 | 5 | 1 | 2 | 0 | 2 | 5 | 19 | 10 | 2 | 15 | 21 | 36 (12) |
| 21-30 | 0 | 14 | 8 | 4 | 14 | 9 | 2 | 0 | 1 | 0 | 0 | 0 | 6 | 25 | 19 | 2 | 25 | 27 | 52 (17.3) |
| 31-40 | 0 | 49 | 19 | 2 | 0 | 0 | 10 | 1 | 12 | 1 | 5 | 0 | 18 | 49 | 28 | 4 | 46 | 53 | 99 (33) |
| 41-50 | 0 | 31 | 17 | 1 | 0 | 0 | 11 | 1 | 6 | 1 | 1 | 0 | 16 | 32 | 19 | 2 | 35 | 34 | 69 (23) |
| 51-60 | 0 | 9 | 5 | 0 | 0 | 0 | 4 | 3 | 7 | 5 | 2 | 1 | 10 | 16 | 8 | 2 | 18 | 18 | 36 (12) |
| >60 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 3 | 3 | 0 | 5 | 3 | 08 (2.7) |
| Total | 0 | 105 | 53 | 7 | 24 | 21 | 31 | 11 | 28 | 9 | 8 | 3 | 57 | 144 | 87 | 12 | 144 | 156 | 300 (100) |
| Total (%) | 105 (35) | 60 (20) | 45 (15) | 42 (14) | 37 (12.3) | 11 (3.7) | 201 (67) | 99 (33) | 300 (100) | |
M: Male; F: Female
Of the 300 onychomycosis cases, only eight cases had associated Tinea manuum and none had Tinea corporis, Tinea pedis or Tinea cruris.
Of the total cases, 238 (79.3%) were positive by direct microscopy and 100 (33.3%) were established by culture [Table/Fig-4].
Comparison of microscopy with culture.
| KOH mount | Culture positive | Culture negative | Total |
|---|
| KOH Positive | 94 | 144 | 238 |
| KOH Negative | 6 | 56 | 62 |
| Total | 100 | 200 | 300 |
Of the total 100 culture positive cases, majority was caused by yeast (66%) and the rest were either due to moulds (24%) or dermatophytes (10%). Amongst the yeasts, Candida albicans (52/66, 78.8%) was the most common species isolated [Table/Fig-5].
Spectrum of fungi isolated (n=100).
| Fungal isolates | Number n (%) |
|---|
| Yeasts- 66/100 |
| Candida albicans | 52 |
| Candida tropicalis | 2 |
| Candida parapsilosis | 2 |
| Trichosporon spp. | 4 |
| Geotrichum spp. | 6 |
| Moulds- 24/100 |
| Aspergillus niger | 16 |
| Aspergillus fumigatus | 6 |
| Penicillium spp. | 2 |
| Dermatophytes- 10/100 |
| Trichophyton rubrum | 10 |
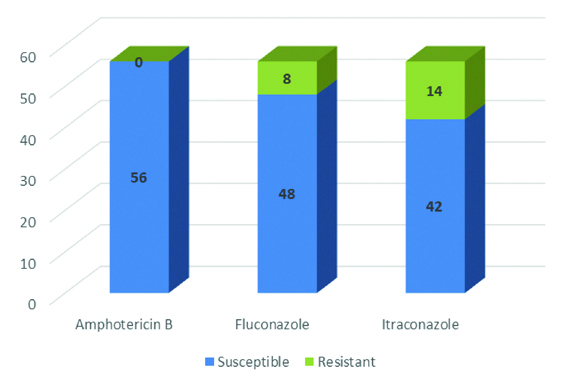
In-vitro susceptibility of Candida isolates showed maximum susceptibility towards amphotericin B (100%). An 85.7% (48/56) isolates were sensitive to fluconazole and 75% (42/56) were sensitive to itraconazole [Table/Fig-6].
Antifungal susceptibility of Candida species.
Discussion
Onychomycosis, the fungal infection of nail plate, though not a fatal disease is still clinically relevant due to its interference with patient’s quality of life, and compromising the functional capacity. Of the 300 onychomycosis cases, in this study, age group most affected was 31-40 years (99/300, 33%) which was in accordance with Gopi A et al., and Veer P et al., [4,9]. A 73.3% of the total cases were in the age from 21-50 years. The predominance of infection in these age group was perhaps due to outdoor activity, prolonged use of shoe in high humidity and repeated nail micro injury.
In this study, females outnumbered the males in contrast to other studies who had reported males to be affected more than females. Fingernails alone were affected in 67% (201/300) of cases and toenails in 33% (99/300) of cases. Fingernails were involved predominantly in females (144/201, 71.7%) which was statistically significant with p-value <0.05. Present study findings are supported by Kaur R et al., who reported 65% fingernail involvement among which 75% were females [10]. This may be due to greater household activity, detergent exposure and prolonged hand wetting and handling organic matter [11].
Present study has evaluated the classical laboratory methods to confirm the diagnosis of clinically suspected cases of onychomycosis. Of the 300 cases in this study, 238 (79.3%) were positive by direct microscopy and 100 (33.3%) were culture positive. Direct microscopy has higher sensitivity than the culture for fungal detection and is statistically significant (p<0.05). In this study positive rate by microscopy was higher to the findings as described by Kaur R et al., and Souza PR et al., where it was 34% and 54%, respectively [3,12]. Prevalence rate of established onychomycosis cases was at lower side (33%) in this study than the studies conducted by various authors from different regions in India [3,13-18] where it varied from 31.3-54% [Table/Fig-7]. Low positive culture rate may occur due to either irregular fungal distribution in the lesions or due to extensive keratinisation as it makes improper collection of nail material resulting in absence or scarce of fungal spores in the sample. Mycological tests performing in three consecutive samples collected at intervals of 2 to 5 days is proven to increase diagnostic accuracy [19].
Prevalence of onychomycosis across India [3,13-18].
| Author | Year of publication | Area | Prevalence rate |
|---|
| Present study | 2020 | Eastern India | 33.3 (%) |
| Chongtham U et al., [13] | 2018 | North-East India | 31.32 (%) |
| Kulkarni S et al., [14] | 2017 | Western India | 54.0 (%) |
| Manash D et al., [15] | 2016 | North-East India | 32.0 (%) |
| Satpathi P et al., [16] | 2013 | Eastern India | 33.7 (%) |
| Niranjan HP et al., [17] | 2012 | South India | 45.0 (%) |
| Kaur R et al., [3] | 2007 | Central India | 45.0 (%) |
| Gupta M et al., [18] | 2007 | North India | 37.6 (%) |
In this study, yeast was the most common isolate (66%) and the rest were the moulds (24%) and dermatophytes (10%). The finding was different from other studies. Veer P et al., reported 29.6% of dermatophytes and 13.6% of non-dermatophytes [9]. In this study T. rubrum was the only dermatophyte isolated. Worldwide among the dermatophytes, T. rubrum is the most common species involved [4]. Amongst the yeasts, Candida albicans (52/66, 78.8%) was the most common species isolated. The finding is comparable to finding by Veer P et al., and Daniel CR [9,20]. Candida albicans is a normal flora but increased prevalence of nail infection by C.albicans is perhaps due to local environmental factors like high temperature and humidity, contact with substances with high carbohydrates and hyperhydrosis [19].
Treatment of onychomycosis has been attempted throughout the ages, but success has been limited until the current decade. In this study, Candida isolates showed maximum susceptibility towards amphotericin B (100%); 85.7% and 75% towards fluconazole and itraconazole, respectively. In a study, addition of amorolfine to oral itraconazole pulse therapy showed beneficial effect in the treatment of Candida fingernail onychomycosis [21]. Fluconazole is a triazole evolved in early 1990s. It is an active antifungal against Candida spp, dermatophytes, and certain other fungi. To treat onychomycosis, fluconazole expresses high chances of cure but long term treatment is required along with demonstration of some potentially significant drug interactions [22]. Itraconazole is highly effective in the treatment of dermatophyte, yeast and some non-dermatophytic mould infections of the nails. Itraconazole has been checked in intermittent dosing or pulse therapy regimes which consist of doses for one week (pulse) per month for a set number of months as it persists in the nail after discontinuation of drugs [23]. Griseofulvin and terbinafine are the other agents available for treatment and terbinafine is considered as the most active drug both in-vivo and in-vitro as per a published study [19]. Onychomycosis is very common in this region where hot and humid climate conditions play an important role in the growth of fungi. Overall, the present study showed the emergence of Candida species as the most common cause of onychomycosis in this region. Despite low fungal isolation rate, fungal culture and antifungal susceptibility testing is utmost importance to know the major fungal aetiology and drug resistance pattern in the locality for better management of onychomycosis case.
Limitation(s)
As in this study, fungi isolated were low in numbers; further study with large sample size is required for a better inference by doing speciation of fungi responsible for onychomycosis and performing the antifungal susceptibility testing to help the clinician for choosing specific antifungal drugs.
Conclusion(s)
Onychomycosis is a common fungal infection of nails. Its prompt and proper diagnosis is essential. It can affect a wide age group involving both males and females though fingernail involvement was mostly found in females, especially housewives doing greater household work. It is caused by yeast, moulds and dermatophytes. Candida albicans was the most common aetiological agent found in the present study. Although all the Candida strains were susceptible to amphotericin B, still some of them showed resistance to the commonly used antifungals like fluconazole and itraconazole. All suspected onychomycosis cases must be diagnosed properly to establish the causative fungus and to choose the proper antifungal drug for effective treatment.
M: Male; F: Female