Epilepsy is a most common chronic neurological disorder. It has a direct impact on children’s lives. People with Epilepsy (PWE) are always at risk of poor QOL. In epileptic children recurrent seizures disturb the consciousness, thinking and judgement and behaviour process [1]. QOL is an important component of human development. It is highly subjective matter, which may vary person to person. It depends on personal lifestyle, family life, work satisfaction, health and safety. Epilepsy carries a vast social stigma and social conflicts which results that PWE are living life below standard. Epilepsy influences actions and memory which impact the QOL [2].
Epileptic children’s are more vulnerable to get other diseases. Self-esteem among adolescents with epilepsy has been compromised, which leads to anxiety [3]. QOL is all about, the degree to which an individual feels healthy, comfortable, ready to participate and enjoy life events without any interruption [4]. Epilepsy is considered as most frequently occurred neurologic disorder across the globe with approximately1% of the population being affected by it [5]. As per record, 80% of the people having epilepsy are from the developing country [6]. India is home to large numbers of individuals with epilepsy. India is a country where many people suffer with epilepsy. Epilepsy is considered as second most common neurological condition in India. It is a neurological condition which disturbs patient and his family [7]. Epilepsy is commonly observed in childhood period. Researcher does not have enough information regarding prevalence of epilepsy among children [8]. A study revealed that drug discontinuation in epilepsy, is very common among patient due to fear of side effects of medications and ignorance of drug dose. An Indian study reported 43% discontinuation rate within one year [9].
Many of the study found that epilepsy cases are not equally distributed as per geographical area. The prevalence rates vary from 3 to 11 per 1000 population in India. It is higher in rural area compare to urban area [10]. There are various QOL tools available but very few are able to measure the QOL in term of Indian context. QOL is directly belongs to culture, tradition, education status, residence, and available health facility. Majority of western QOL tools are not applicable in Indian context due to lack of awareness about disease, literacy rate and qualification different treatment strategy, government policy, health care delivery services, higher school drop rate, poor socialisation, social aspects (stigma, marital issue, nonacceptance in family), spiritual aspects, traditional beliefs, black magic, blind faith, family organisation and structure [11]. So, the main aim of this study is to develop and validate QOL tool for Indian epileptic children age between 10-18 year.
Materials and Methods
A mix method study [Table/Fig-1] with sequential exploratory design was conducted. Sequential exploratory where first qualitative data and secondly quantitative Information has been gathered [Table/Fig-2].
Diagrammatic representation of mix method study.
Phase of mix method study.
| Steps | Phase | Output |
|---|
| In-depth interview of participant till data saturation (n=15) | Qualitative phase | Seeking information |
| Coding and thematic analysis by conventional content analysis method | Qualitative phase | Convert information into 84 questions |
| Convert data into scaling | Quantitative phase | Likert scale of 1-5 point where 1=Always5=Never |
| Data collection (n=20) phase II | Quantitative phase | Collect raw score of each item |
| Exploratory data analysis, internal consistency and reliability | Quantitative phase | Descriptive and analytic statistics |
| Integration of quantitative and qualitative data | Final tool development | 45 items in tool includes discussion |
In phase I, qualitative data were gathered from the 15 children those were diagnosed with epilepsy. In phase II, data were collected from 20 epileptic children by using 54 items. The data were collected from Outpatient Department (OPD) of three children hospitals in central part of Gujarat. Each participant were asked question as per interview guideline and responses were noted. Each interview last for 30-40 minutes. The first and second phases of the study were carried out between May 2019 to December 2019, respectively. The study was approved by the Institutional Ethics Committee of CHARUSAT (RPCP-IECHR-2018-19/R-04.01). A written informed consent and assent was taken from parents and children before their participation in both phases.
Inclusion criteria: Children from 10-18 year of age with a known case of epilepsy, atleast had two episode, seizure free at the time of recruitment and willing to participate in the study.
Exclusion criteria: Children with an active psychiatric disorder, congenital anomalies and refusal to give consent.
Statistical Analysis
The statistical analysis was conducted by using SPSS software. Reliability, factor analysis, internal consistency, scree plot, inter item correlation, item total correlation was calculated. The reliability was measured by cronbach’s alpha.
Results
The following steps were followed for tool development.
Phase-I (Qualitative)
In-depth review of literature regarding: Learning through reading is one of the strength of research. Reading stimulates thinking process. Here researcher read various available literature related to tool development, mix method design, factor analysis, existing QOL tool, misconceptions about epilepsy and case studies of epilepsy [12-14].
Participant’s interview: The primary step in tool development is item pool generation. A researcher can generate items on the basis of experience, literature review and information received from the participants. The investigator conducted in-depth interview of epileptic children. The process of interview continues till the data saturation. Total 15 participants were involved in this phase. Each interview last for 30-40 minutes as per interview schedule [Table/Fig-3]. The interview was recorded and written for the verbatim transcripts in English Language. The transcripts were then analysed using conventional content analysis. Here investigator extracted contextual category, themes and significant statement. Data were collected and analysed through Colaizzi method [15].
Interview schedule for epileptic child.
| S. No. | Questions | Response |
|---|
| 1 | Tell me something about your illness like when it started and how it was diagnosed? | |
| 2 | Share your experience of last epileptic seizure? | |
| 3 | How it affect your social life like behaviour, communication, relation with friends? | |
| 4 | How it affect your physical function like require more supervision, toilet, walking, climbing? | |
| 5 | How it affects cognitive function like learning, memory, concentration, remembrance, judgment? | |
| 6 | How it affect emotion like mood changes, anxiety, and thinking? | |
| 7 | What problem you are facing because of epilepsy at home? | |
| 8 | What problem you are facing because of epilepsy at your school? | |
| 9 | Your health is better than previous or worse? | |
| 10 | Describe the effect of antiepileptic drug. | |
Expert opinion- Result of participant’s interview was discussed among expert those were medical practitioners, certified nurse’s and care taker of children. Medical practitioner’s suggested to incorporate questions related to the physical activity and cognitive domain. Nurses suggested to include item related to children’s behaviour, psychosocial wellbeing and communication. Care taker suggested covering sleeping behaviour of child after episode. As per the advice of experts, correction was imposed.
At the end of this phase, a pool of 84 items with five domains was evolved [16]. Individual items identified by the three parallel approaches namely expert opinion, patient interview and review of existing literature were then formulated into specific questions and screened for duplicate items. Overall, 20 items were omitted due to duplicity and irrelevance. Language of many items was modified. It would be implemented in phase II.
Phase-II (Quantitative Phase)
In this phase, 54 items administered to 20 epileptic children diagnosed with epilepsy. An instruction about filling of questionnaire, timing, scoring and evaluation was conveyed to participants by researcher. The questionnaire was in local Gujarati language.
Inclusion criteria: The participants who fullfilled the inclusion criteria were involved into study. The inclusion criteria for both phase that child from 10-18 year of age with a known case of epilepsy, at least had two episode, seizure free at the time of recruitment and also willing to participate in the study.
Exclusion criteria: The exclusion criteria were children with an active psychiatric disorder and other congenital anomalies and refuse to give consent.
Baseline information including age of child, gender, family history of epilepsy, average duration of epilepsy, occurrence of epilepsy, number of times admitted in hospital due to epilepsy, Current treatment was collected. Validity of tool was calculated by using different methods.
Validity: The validity of a research study refers to how well the findings of the study reflect true results among similar people outside the study [17].
Face validity: Face validity is the degree to which a measure seems to be connected to a particular construct [18]. It is simplest type of validity. The face validity of tool was found satisfactory.
Content validity: Content validity means measure the content of an instrument and identify, whether it measures all the characteristics of construct appropriately. Here researcher used I-CVI and S-CVI for content validity. It is the checking of the relevance for content as per domain for the tool. Total 15 experts like neurologist, clinical psychologist, and researcher of tool development study were involved in content validation. The researchers asked the panel of experts to give their viewpoints on the items generated through phase-I for the construct of epilepsy. The CVI was calculated for all items in terms of I-CVI and S-CVI. For CVI, the panel of expert was asked to rate each scale items in terms of its relevance to the underlying construct. A 4-point scale was used to avoid a neutral point. The four points in the item rating indicates 1=not relevant, 2=somewhat relevant, 3=quite relevant, 4=highly relevant. For each item, I-CVI was computed as the number of experts giving a rating of 3 or 4, divided by the total number of experts.
The S-CVI was computed for ensuring content validity of the overall scale. The content validity of tool was done by a series of interaction with the expert and on the basis of agreement. After getting the suggestion from the experts and incorporating it, a total of 10 items were removed due to the poor agreement index. Many of the items were modified as per expert suggestion. A new draft of the item was again sent for content validation to experts. This process of validation continued till individual I-CVI reach to the level of acceptance that is 0.8. The S-CVI was calculated and found more than 0.80 which is acceptable in range.
Construct validity: Construct validity evaluates whether a measurement tool really represents the thing, which researcher is interested in measuring.
Response options: The 5-point rating scale was selected to maximise the capacity of the scale to measure change. All items were assessed e.g., Always (1), frequently (2), often (3), very rare (4), never (5). Here ‘1’ indicated poorest QOL for choices like ‘always’ and the highest rating of ‘5’ denoted the best QOL standing for ‘never’.
Item analysis: The following tests were used for analysis of data. Exploratory factor analysis, Factor loading, Inter item matrix and Item to item total score.
Second draft of questionnaire (54 items) was administered to 20 samples. Participants were recruited after an informed written consent from parents. Participants were diagnosed with epilepsy and the age was between 10-18 year. The corrected item total correlation is important indicator in the process of tool development [19].
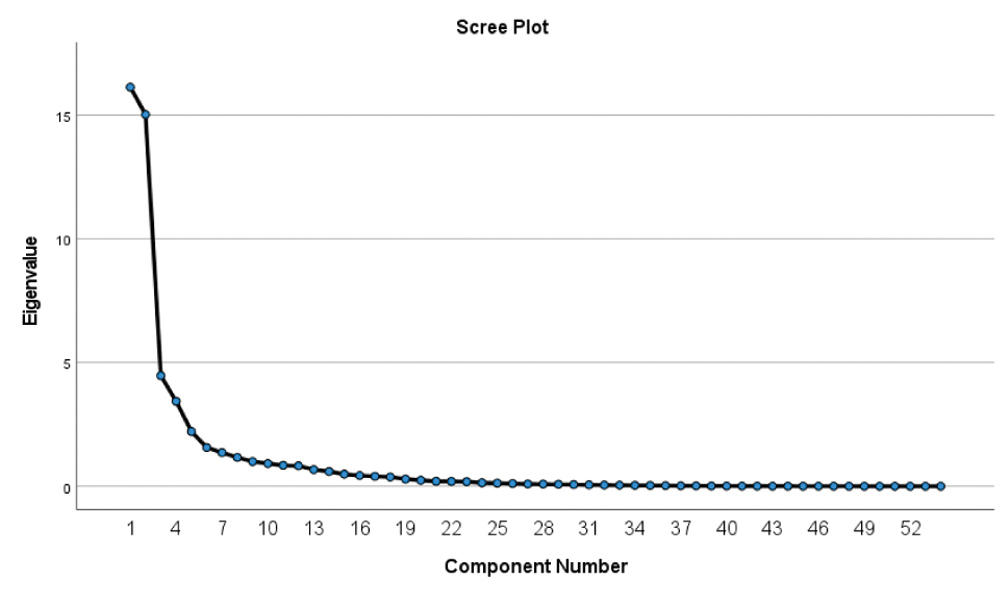
As per [Table/Fig-4], all 54 items were classified into 5 factors as per factor analysis matrix. These five domains (named physical endurance, social and communication, psychological, cognitive aspects and treatment) covered 76.42% of variance. Scree plot showing five components on the basis of eigen value for all 54 items, as per statistical analysis [Table/Fig-5].
| Component | Initial eigen values | Rotation sums of squared loadings |
|---|
| Total | % of variance | Cumulative % | Total | % of variance | Cumulative % |
|---|
| 1 | 16.131 | 29.872 | 29.872 | 15.688 | 29.051 | 29.051 |
| 2 | 15.027 | 27.828 | 57.700 | 14.183 | 26.266 | 55.317 |
| 3 | 4.468 | 8.274 | 65.974 | 4.542 | 8.412 | 63.728 |
| 4 | 3.433 | 6.357 | 72.331 | 4.452 | 8.244 | 71.972 |
| 5 | 1.612 | 4.097 | 76.427 | 2.406 | 4.455 | 76.427 |
Scree Plot showing various factors.
Item reduction (Principal component analysis)-Data from draft of 54 items was statistical evaluated. Items were deleted on the basis of item total correlation and inter item correlation matrix. The [Table/Fig-6] showing the range of score considered as acceptable. If the calculated value is not in acceptable range, then that item would be removed from construct.
Range consider as acceptance.
| Analysis | Acceptable value |
|---|
| Cronbach’s alpha | >0.7 |
| Inter item correlation | <0.7 |
| Corrected item total correlation | 0.2 to 0.8 |
Reliability analysis- To measure the internal consistency of the tool item, the Cronbach’s alpha was calculated. It helps in measurement of the reliability of tool.
Inter-item correlation- It is a way of evaluating the reliability of internal accuracy. It is a measure that individual questions provide clear, suitable results on a test or questionnaire.
As per item-total correlation, in this study, five items which had item total correlation coefficient of <0.3 were excluded while four items were excluded as they were showing an inter item correlation of >0.7 [Table/Fig-7]. So, total nine items were deleted.
| Factor | Item no. | Item | Reason for exclusion | Changes in cronbach’s alpha if item deleted |
|---|
| Factor 1 | Physical 10 | You experience increase heartbeat and sweating when you see sudden heavy fire during HoliDahan and Diwali. | Item total correlation <0.3 | 0.912 |
| Factor 1 | Physical 12 | You experience vertigo when flying kite (At utrayan) and for long period (>3 hours). | Item total correlation <0.3 | 0.938 |
| Factor 2 | Social 11 | You become unhappy, when there is family gathering at your home. | Item total correlation <0.3 | 0.898 |
| Factor 3 | Psychological 4 | You feel that Loud music during Navratra, Ganesh festival, Dashamata or religious festival causes seizure | Inter tem correlation >0.7 | 0.923 |
| Factor 3 | Psychological 8 | You become tensed and depressed | Inter tem correlation >0.7 | 0.915 |
| Factor 4 | Mental function 11 | You feel difficulty in understanding the directions like East, West, North, South, right, left, up, down, ascending, descending, vertical, horizontal etc | Item total correlation <0.3 | 0.863 |
| Factor 4 | Mental function 12 | You get confused with the same words like I, Eye, By, Buy, Bye, Read, Red, lose, loose, loss, lake, lack, lac etc., | Item total correlation <0.3 | 0.884 |
| Factor 5 | Treatment and medicine 4 | You feel embarrassed because you need to take medications | Inter tem correlation >0.7 | 0.872 |
| Factor 5 | Treatment and medicine 6 | You believe that medication cannot recover epilepsy | Inter tem correlation >0.7 | 0.887 |
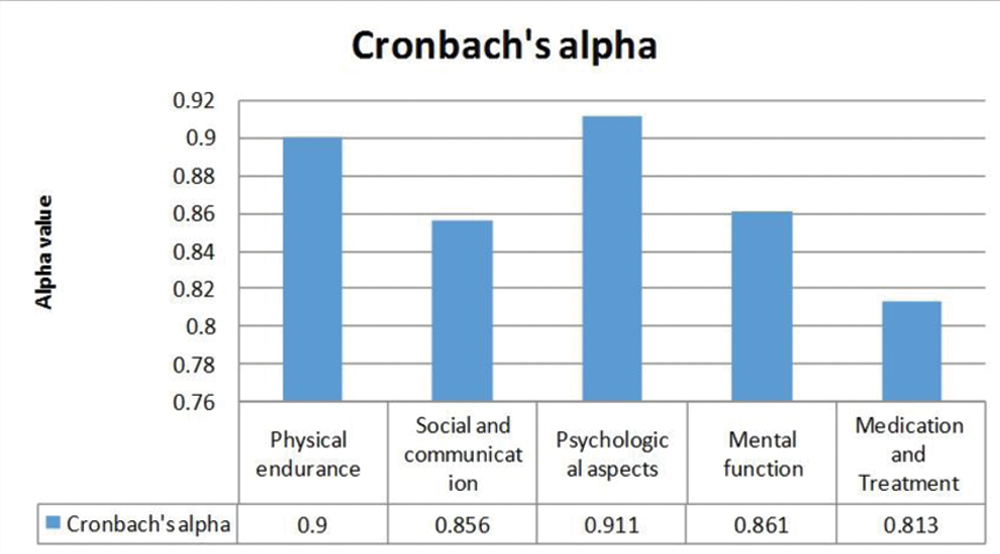
Reliability- Internal consistency reliability (Cronbach’s alpha)- The value of alpha above 0.7 is acceptable. Cronbach Alpha value of each component was calculated [Table/Fig-8] and found satisfactory.
The final draft of tool having five components which covers the following aspects of child life. Each component consists of items. As per [Table/Fig-9] each item measured the response in five point likert scale. So each item showing minimum score one and maximum score five.
Maximum and minimum score of 45 items.
| Component as per domain | No of items | Min | Max |
|---|
| 1. Physical endurance (body function) | 13 | 13 | 65 |
| 2. Social and communication | 10 | 10 | 50 |
| 3. Psychological aspects | 8 | 8 | 40 |
| 4. Mental function (Memory and Understanding) | 10 | 10 | 50 |
| 5. Medication and treatment | 4 | 4 | 20 |
| Total | 45 | 45 | 225 |
All the items were divided in five components as per factor analysis.
Component I (physical domain) had 13 items related to role limitations, play, and lack of sleep and activity of daily life. The reliability of this domain was calculated to 0.90 which is acceptable in range. The inter-item total correlation as per matrix is also seemed satisfactory which varied from 0.013 to 0.870. The Cronbach’s alpha of physical endurance is calculated 0.90 and corrected item total correlation is varied from 0.175 to 0.796.
Component II (social and communication) had 10 items which included relationship, teacher role, family gathering and loneliness. The reliability of this domain was calculated to 0.856 which is acceptable in range. The inter-item total correlation as per matrix is also seemed satisfactory which is ranged from -0.011 to 0.812. The Cronbach’s alpha of this domain is calculated 0.856 and corrected item total correlation is ranged from 0.177 to 0.721.
Component III (psychological aspect) had eight items related to commitment, pessimistic life, worries and carrier opportunity. The reliability of this domain was calculated to 0.911 which is acceptable in range. The inter-item total correlation as per matrix is also seemed satisfactory which is ranged from -0.011 to 0.812. The Cronbach’s alpha of this domain is calculated 0.861 and corrected item total correlation is ranged from 0.177 to 0.721.
Component IV (mental functioning) had 10 items related to understanding, decision making, memory, and concentration. The reliability of this domain was calculated to 0.861 which is acceptable in range. The inter-item total correlation as per matrix is also seemed satisfactory which is ranged from -0.087 to 0.928. The Cronbach’s Alpha of this domain is calculated 0.861 and corrected item total correlation is ranged from 0.136 to 0.900.
Component V (medications and treatment) had 4 items related to drug and its side effect, treatment burden on family. The reliability of this domain was calculated to 0.813 which is acceptable in range. The inter-item total correlation as per matrix is also seemed satisfactory which is ranged from 0.169 to 0.610. The Cronbach’s alpha of this domain is calculated 0.813 and corrected item total correlation is ranged from 0.414 to 0.679.
Discussion
The tool developed and validated consists of 45 items which cover five domain as per factor analysis which are physical endurance (13), social and communication (10), psychological aspects (8), mental functioning (10) and treatment and medication (4). All these domains and items had high internal consistency (Cronbach’s alpha 0.90). The newly developed tool is concerned to be a most valid and reliable tool which measures the QOL among children who suffered with epilepsy. Health personnel’s will be motivated to utilise this series of question (questionnaire) for routine examination of epileptic client. It able to find the effects of the disease progress and its impact on well-being for epilepsy patients. The steps of QOL tool development are important. Similar steps were followed in a study carried out at Chennai, South India with title “Validation of QOL questionnaire for patients with cancer in Indian perspectives”. There were various oncological tools available but due to geographical boundaries, a common solution may not be applicable and hence there is a need to develop a regional based tool and validate (standardise) the same tool. At first phase 38 items were pooled from currently available tools, reviews of case studies, and the field trial. Principal component method with varimax rotation was used. Cronbach alpha coefficient was used for reliability analysis. Total 10 factors were identified through factor analysis. The eigen value on graph range from 1.10 to 8.55. All 10 components cover 62.6% variance. The first or primary factor contributed 22% variance. The factors were named as psychological well being, bodily well being, self confidence, support from outside and many more. The value of Cronbach alpha was measured 0.9 which shows good internal consistency of tool. The tool’s split-half reliability was 0.74. Overall the newly developed tool was found highly reliable for cancer patient [20].
The study is supported with Taylor RS et al., that there are variety of epilepsy related tools are available like Quality of Life in Epilepsy-31 (QOLIE-31), Quality of Life in Epilepsy-10 (QOLIE-10), Quality of Life in Epilepsy Inventory-89 (QOLIE-89). Some tools were related to parents and some were related to young people. There are scarcity of HRQoL (Health related Quality of Life) measures for specific epilepsy groups such as children, adult and client with learning disability [21].
Many of the frequently used tool missing the information which is important in Indian context tool [Table/Fig-10]. This study result is different from paediatric QOL inventory. In childhood and adolescence, numerous instruments are currently available to calculate HRQOL. There is a generic core scale and multiple disease specific scales for the paediatric QOL inventory. The information was gathered from children aged between 2 to 18 years of California, USA. Now, it is applied on a worldwide QOL scale. The concept of QoL is likely to be influenced by a number of cultural, social, religious, ethnic and geographic variables and can be viewed differently by children and parents from different backgrounds. The mean total HRQOL score listed was 83.8 (12.7) in a study conducted among children from the USA, while a study among Thalassemia children from Thailand recorded a score of 76.7 (11.4), both from child self-reports [22]. So, a local based tool is better than global based tool. Even paediatric QOL inventory does not include the impact of treatment and medication on child life. So, the present study can say that this scale is more reliable compare to other available tools. There are very few QOL tool available in Indian context. This newly developed tool will help the health care provider to assess the QOL of epileptic children which further help in treatment.
Existing QOL tool and participant information.
| Patient identified issues | Information available in existing toolsYes/No |
|---|
| Information | QOLCE-76 | QOLIE-AD-48 | QOLIE-31 | QOLCE-55 | QOLIE-89 |
|---|
| Discourage by relatives and neighbours | No | No | No | No | Yes |
| Misconception towards the treatment | No | No | No | No | No |
| Forced to play indoor | No | Yes | No | No | No |
| Limited activity in dance, Garba | No | No | No | No | No |
| Vertigo during wedding due to crowd | No | No | No | No | No |
| Well of death/merry go round | No | No | No | No | No |
| Western toilet and comfort ability | No | No | No | No | No |
| School drop | No | No | No | No | No |
| Navratra and lack of sleep | No | No | No | No | No |
| Dashamata and heavy DJ sound | No | No | No | No | No |
| Vehicle LED & bright light trigger episodes | No | No | No | Yes | No |
| Elevators and seizure | No | No | No | No | No |
| Teacher’s understanding about epilepsy | No | No | No | No | No |
| Feel embarrassed due to epilepsy | No | No | No | No | Yes |
| Competition in study at school | No | No | No | No | No |
| Confusion in right, left, up down | No | No | No | No | No |
| Parents income and treatment | No | No | Yes | No | No |
| Schooling and leave | No | No | No | No | No |
QOLCE-76: Quality of life in children with epilepsy; QOLIE-AD-48: Health-related quality of life for adolescents-48 item; QOLIE-31: Quality of life in epilepsy-31; QOLCE-55: Shortened Quality of Life in Childhood Epilepsy Questionnaire-55; QOLIE-89: Quality of life in epilepsy inventory-89
Limitation(s)
The study sample was limited to central region of Gujarat. Subjects were recruited on the basis of non-probability sampling, age of children 10-18 year and data collected from OPD of hospital.
Conclusion(s)
Newly developed QOL (45 items) is a reliable, valid and sensitive tool for the assessment of QOL of Indian epileptic children. Further research studies are required to validate the tool for application in other states and wider socio-economic community settings. Linguistic validation is also needed to administer in different languages with more number of participants. This tool can be utilised to find out psychosocial life of epileptic children, which further can be useful in counselling of such children.
QOLCE-76: Quality of life in children with epilepsy; QOLIE-AD-48: Health-related quality of life for adolescents-48 item; QOLIE-31: Quality of life in epilepsy-31; QOLCE-55: Shortened Quality of Life in Childhood Epilepsy Questionnaire-55; QOLIE-89: Quality of life in epilepsy inventory-89