Malaria is a highly complex disease with protean manifestations. Prolonged infections can lead to severe anaemia, metabolic acidosis, haemoglobinuria, splenomegaly, hepatomegaly, and other severe illnesses. P.falciparum infection is known to cause single and multiorgan related fatal conditions, including cerebral malaria, renal failure, hepatic dysfunction and failure, and ARDS [9].
Hypocalcaemia has established itself to correlate with the complications in this parasitic disease [10]. The mechanism of hypocalcaemia in malarial disease has not been explained, but various theories have been postulated. The plasmodium is found to use calcium signalling pathway and it increases the RBC’s permeability to calcium. This then increases the calcium influx and thus reduces calcium levels [11]. There is inadequate data supporting the role of hypocalcaemia in evaluating the severity of the disease [12].
Due to the high incidence of this parasitic disease in Mangaluru and the scarce data on the clinical significance of hypocalcaemia in malaria, this study was done to correlate hypocalcaemia with the complications in malaria.
Materials and Methods
This was a cross-sectional, observational study conducted in a tertiary care centre from January 2018 to June 2019. The study was approved by Father Muller Institutional Ethics Committee (FMMCIEC/CCM/09/2018). A written informed consent was obtained from all the patients participating in the study. All adult patients ≥18 years of age, presenting with acute fever (oral body temperature >38°C) diagnosed to have malaria by peripheral smear and admitted to the medical wards and intensive care unit were included in the study.
The study excluded patients on calcium supplementation, drugs causing hypocalcaemia and those having pre-existing kidney or liver disease. Sample size was calculated using the formula
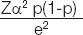
Zα=1.96 at 95% Confidence Interval; p=17/60=28% [10]; e=10%. Hence, a sample size of 75 was considered.
A detailed history with a special importance to the complications was taken followed by a thorough general physical examination and a systemic examination to document the findings in a structured proforma, which included the symptomatology, type of malaria, various complications, necessary laboratory data such as haemoglobin, platelet count, liver and renal functions serum electrolytes, chest X-ray and arterial blod gas analysis.
Patients were classified as having complicated malaria if they met atleast one of the following criteria as per World Health Organisation (WHO): Hypoglycaemia (<40 mg/dL), Metabolic acidosis (HCO3 <15 mmol/L or Lactate >5 mmol/L with rapid, deep and labored breathing), Haemoglobin <7 g/dL or Haematocrit <20%, significant bleeding including recurrent or prolonged bleeding from nose, gums or venepuncture sites; hematemesis or melaena, prostration (Generalised weakness so that a person is unable to sit, stand, or walk without assistance). Jaundice: Serum bilirubin >3 mg/dL, Renal failure (S. Creatinine >3 mg/dL or S.Urea >60 mg/dL), Pulmonary oedema (Radiological, oxygen saturation of <92% on room air, respiratory rate >30/min, severe chest indrawing and crepitations on auscultation), Shock (Systolic BP <80 mm of Hg with cold peripheries and prolonged capillary refill time) and impaired consciousness with Glasgow coma scale <11 [13].
All the patients underwent confirmatory test for malaria using peripheral smear. Other routine tests included complete blood count, renal function tests, blood sugar levels, liver function tests, chest X-ray and standard 12 lead electrocardiogram.
Serum calcium level was estimated using NM-BAPTA technique [14] and blood samples were obtained without using tourniquet. Patient were classified as having hypocalcaemia if serum calcium level is <8.4 mg/dL (The normal range of serum calcium considered as 8.4-10.2 mg/dL) [15]. Serum albumin was estimated and corrected calcium was calculated using the formula S.Ca+{(4 -S.Albumin) × 0.8}. Repeat serum calcium was done in patients who recovered from complications and was correlated accordingly. QTc prolongation was calculated using the formula (QT/√RR). The patients diagnosed with malaria were grouped into complicated or uncomplicated, and the calcium levels were compared among the two groups.
Statistical Analysis
Descriptive statistics were used to define characteristics of the study variables by showing mean, dispersion (standard deviation) and distribution by showing counts and percentages. Chi-square test and student t-test were used to establish the relationship between calcium levels, QTc prolongation and complications. A conventional p-value <0.05 was the rejection criteria for the null hypothesis and was considered significant. Sensitivity/specificity and Receiver Operating Characteristic (ROC) curve analysis was done to determine the relationship between calcium levels and complications. This study was analysed using SPSS version 23.
Results
During the study period, a total of 75 patients who met inclusion criteria were included in the study. In this study, majority of the patients were young (21-30 years) with a mean age of 38.4±8.2 years [Table/Fig-1]. In the present study 53 cases (70.7%) were males and 22 cases (29.3%) were females, with a M:F ratio of 2.4:1. Among the patients, majority had vivax malaria (41 patients, 54.6%) followed by falciparum (30 patients, 40%) and mixed (4 patients, 5.3%).
Age distribution of the patients.
| Age distribution (Years) | Number of patients (%) |
|---|
| 18-20 | 16 (21.33%) |
| 21-30 | 23 (30.67%) |
| 31-40 | 6 (8.00%) |
| 41-50 | 12 (16.00%) |
| 51-60 | 10 (13.33%) |
| 61-70 | 5 (6.67%) |
| 71-80 | 3 (4.00%) |
| Total | 75 |
In the present study, 37 patients (49.3%) had low serum calcium levels on admission. After calculating the corrected calcium, hypocalcaemia was present among 25 patients (33.3%). The association of age and hypocalcaemia was not statistically significant with a p-value of 0.691 by Chi-square test. Among the patients with hypocalcaemia, majority of them had P.vivax malaria. However, this was not statistically significant with a p-value of 0.320 [Table/Fig-2]. The laboratory profile of patients is shown in [Table/Fig-3].
Serum calcium levels in different types of malaria.
| Malaria parasite smear | Ca level | Total |
|---|
| <8.4 (mg/dL) | 8.4-10.2 (mg/dL) |
|---|
| Count | % | Count | % | Count | % |
|---|
| Falciparum | 11 | 37.9 | 18 | 62.1 | 29 | 100 |
| Mixed | 0 | 0 | 4 | 100 | 4 | 100 |
| Vivax | 14 | 33.3 | 28 | 66.7 | 42 | 100 |
| Total | 25 | 33.3 | 50 | 66.7 | 75 | 100 |
Chi square test p=0.320, Not significant
Laboratory profile of study participants.
| Parameter | Number of patients (N=75) |
|---|
| Haemoglobin |
| <7 gm/dL | 0 (0%) |
| 7-10 gm/dL | 10 (13.3%) |
| 10-12 gm/dL | 35 (46.6%) |
| >12 gm/dL | 30 (40%) |
| Platelet count |
| <1,00,000/mm3 | 40 (53.3%) |
| 1,00,000-1,50,000/mm3 | 22 (29.3%) |
| >1,50,000/mm3 | 13 (17.3%) |
| Serum creatinine |
| >3 mg/dL | 1 (1.3 mg/dL) |
| 1.3-3 mg/dL | 6 (8%) |
| <1.3 mg/dL | 68 (90.6%) |
| Serum bilirubin |
| >3 mg/dL | 8 (10.6%) |
| ≤3 mg/dL | 67 (89.3%) |
| AST |
| >45 U | 20 (26.6%) |
| 10-45 U | 55 (73.3%) |
| ALT |
| >45 U | 22 (29.3%) |
| 10-45 U | 53 (70.6%) |
In the present study, 18 (24%) of the cases had complications. Among the 25 patients who had hypocalcaemia, 13 (52%) of them had complications during their illness [Table/Fig-4]. It was statistically significant with a p-value of 0.001 by fisher’s-exact test. Hypocalcaemia had a relative risk estimate of 9.75% (CI:2.901%-32.766%) for developing complications. With ROC analysis, the calcium value cut-off for complications was 8.25 with a sensitivity of 72%, specificity of 86%, Positive Predictive Value (PPV) of 61.9% (CI:41.1%-82.7%), Negative Predictive Value (NPV) of 90.7% (CI:83%-98.5%), likelihood ratio of 5.146 (CI:2.546-10.399) and accuracy of 82.7% (CI:74.1%-91.2%) with AUC of 0.797 [Table/Fig-5].
Association of serum calcium levels with complications.
| Complications | Calcium <8.4 mg/dL (N=25) | Calcium 8.4-10.2 mg/dL (N=50) |
|---|
| No complications | 12 (48%) | 45 (90%) |
| Acute kidney injury | 1 (4%) | 0 (0%) |
| Acute respiratory distress syndrome | 1 (4%) | 0 (0%) |
| Cerebral malaria | 1 (4%) | 0 (0%) |
| Haematuria | 1 (4%) | 0 (0%) |
| Jaundice | 3 (12%) | 5 (10%) |
| Multiorgan failure | 1 (4%) | 0 (0%) |
| Shock | 5 (20%) | 0 (0%) |
Fishers exact test p=0.001, Highly significant
ROC curve for hypocalcaemia and complications.
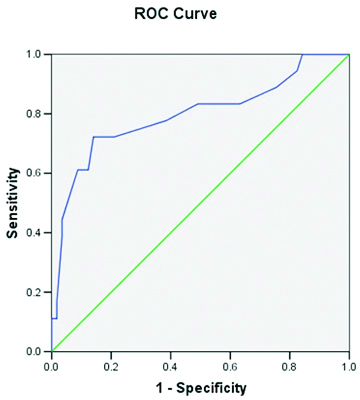
In the present study, only a small subset of 4 cases (5.3%) infected with faciparum had abnormal ECG changes in the form of QTc prolongation. All these patients also had hypocalcaemia. The association of hypocalcaemia and QT prolongation was highly significant with a p-value of 0.001 by t-test. All the patients with QT prolongation had complications and this was statistically significant with a p-value of 0.002 by Fisher’s-exact test. Patients with QT prolongation had a relative risk of 5.071 (3.172-8.109, 95% CI) for developing complications. With ROC analysis, the calcium value cut off for QT prolongation was 7.85 with a sensitivity of 100% and specificity of 99.9% and AUC 0.986.
Among the hypocalcaemic patients having complicated malaria, the serum calcium was repeated after the patient recovered from the complications. The calcium levels reverted to normal in all the patients. However, this was not statistically significant (p-value=0.185).
Discussion
The resurgence of malaria is a serious public health problem in many parts of the world. It is therefore prudent to identify the factors which contribute to susceptibility of hosts. This parasitic disease is a major public health problem in developing countries, especially in India, which contributes drastically to the parasitic disease burden on the whole in Southeast Asia countries [16,17]. India is one of the major contributors to malaria mortality and morbidity in South and Southeast Asia region [17].
In the present study, majority belonged to the young age group which is in comparison with various other studies [18-20]. The age distribution depends on the endemicity of malaria in the geographical area. The comparison studies were also conducted in malaria endemic areas.
In our study, there was a male preponderance which is in comparison with various other studies [21,22]. The male predominance could be due to prolonged exposure of men to infected mosquitoes during outdoor working hours.
In the present study, majority of the patients had vivax, which is comparable to a recent large population study [23]. This is in contrast to another Indian study where majority of the patients had falciparum malaria [24]. The type of malaria depends on the geographical location and as vivax predominates over the other types of malaria in our area, this could explain the difference [23].
In the present study, hypocalcaemia was present in nearly one-third of the patients, among whom half of them had complications. Our observations were comparable to study done by Petithory JC et al., where hypocalcaemia was evident in 33% of the patients with 60% of them having complications [25]. However, another study reported a higher percentage (42%) of hypocalcaemia as compared to our study. This could be due to larger sample size in the former study [26]. Similarly, in a study done in South India where the sample size was comparable, hypocalcaemia was present in nearly half of the study subjects (45%) [10]. Hypocalcaemia was documented in a strikingly large number of complicated malaria cases (88.2%) in their study. This percentage was marginally higher as compared to the present study. The reason could be due to slightly higher percentage of the complications in the former study. The comparison study was conducted nearly two decades ago, since which time there has been a significant improvement in hospital care, patient awareness about the disease with early detection and timely treatment thus lessening the complications and mortality.
In a study done by Maitland K et al, hypercalcaemia was found to be more common in malaria [27]. This result was not comparable to our study as we did not have any patients with hypercalcaemia. This could not be explained and was deemed as incidental. Further studies need to be done to validate the same.
In the present study, normalisation of serum calcium level was seen in majority of hypocalcaemic patients after they recovered from the complications. Similar finding was documented in a study which showed that return of calcium levels to normal coincided with clinical recovery and parasite clearance [10]. Although there is a mention of hypocalcaemia as a feature of complicated malaria, there is no literature on its prevalence and clinical implications. Severity of hypocalcaemia in malaria was found to correlate with heavy parasitaemia and complications. Hence, hypocalcaemia in malaria may be a biochemical marker for complications. Hypocalcaemia secondary to parathormone suppression is another entity which was not studied and can possibly explain the reason behind this abnormality [10,28]. Recovery of hypocalcaemia correlates with improvement of parathyroid glandular function when parasitaemia gets cleared. Other proposed hypothesis for hypocalcaemia in malaria includes hypophosphatemia and hypomagnesemia [10].
In the present study, QTc prolongation was observed in a small subset of patients with falciparum malaria who had hypocalcaemia and complications. This was slightly low as compared to study done by Mananje SR et al., where 13% of the patients had QTc prolongation [18]. The difference could be due to larger number of study subjects in the comparison study. However, majority of the patients with QTc prolongation had complications similar to our study. QTc prolongation may be due to sequestration of the parasite in the myocardium.
In a prospective study, QTc prolongation was seen in nearly half of the cases of complicated malaria (53.8%) [24]. This is higher compared to our study where 22.2% of the complicated malaria had QTc prolongation. The reason for this difference is probably due to larger study participants and higher percentage of complications in the comparison study as compared to our study.
An Indian study showed that out of the 27 patients with hypocalcaemia, QTc prolongation was seen in 11 patients (40%) [10]. This is higher as compared to our study where 16% of hypocalcaemic patients had QTc prolongation. One of the possible explanations for the difference could be due to the judicious use of newer antimalarials for treatment of malaria and reduced use of quinine which can cause QTc prolongation.
Limitation(s)
The study was limited by a small sample size. The exact cause of hypocalcaemia in the study could not be ascertained.
Conclusion(s)
Hypocalcaemia had a significant correlation with the complications in malaria. Serum calcium level should be estimated early in the course of the illness to predict the future complications in malaria.
Chi square test p=0.320, Not significant
Fishers exact test p=0.001, Highly significant