Enterobacteriaceae are frequently encountered pathogens in community and healthcare acquired infections [1]. Resistance mechanisms like Extended Spectrum Beta Lactamase (ESBL) production to commonly available antimicrobial agents is a well-recognised problem amongst members of Enterobacteriaceae and Carbapenems have served an important antimicrobial class to treat infections caused by these strains [1,2]. However, resistance to the Carbapenem group of antimicrobial agents through production of various Carbapenemases like KlebsiellaPneumoniae Carbapenamase (KPC), New Delhi Metallobetalactamase (NDM), Verona Integrin-encoded Metallobetalactamase (VIM), Imipenemase (IMP), and Oxacillinase (OXA) is being increasingly reported [1].
Centre for Disease Control and Prevention (CDC) defines CRE as any family member of Enterobacteriaceae resistant to imipenem, meropenem, doripenem or ertapenem [3]. Enterobacteriaceae producing carbapenemases are also resistant to other beta lactam group of antimicrobial agents, thereby leaving a very few treatment options such as polymyxins and tigecycline [4]. In view of limited treatment options available for treatment of infections such as urinary tract infections, pneumonia, bloodstream infections and skin and soft tissue infections caused by CRE and to prevent further spread of these organisms, healthcare systems across the world have measured the magnitude of the problem. CDC has described an increase of prevalence of CRE from 1 to 4% in 2013 [1]. High, up to 50%, mortality rates in CRE infections makes it a serious global public health threat [1,5]. Keeping in mind the therapeutic challenge of infection by CRE, high morbidity, mortality and potential to spread in healthcare setting, measuring the magnitude of CRE becomes significant and thus present study was conducted to generate data on CRE from this part of Gujarat, India.
Materials and Methods
This retrospective observational study included five years data from January 2014 to December 2018. The study was duly approved by Institutional Ethics Committee (IEC/HMPCMCE/Ex. 16/82/17). The data required for determining the prevalence of CRE was obtained from the LIS of Central Diagnostic Laboratory at Shree Krishna Hospital and was analysed in 2019.
Inclusion and Exclusion criteria: All isolates belonging to the Enterobacteriaceae family and isolated from patients’ specimen sent for culture, were included in the study. The isolates that were obtained in duplicate from the samples collected from the same site of infection were considered as a single isolate and were excluded from the study.
Study Procedure
The identification and antimicrobial susceptibility of the Enterobacteriaceae was performed using Vitek 2 automated system in Microbiology laboratory. The susceptibility patterns of all Enterobacteriaceae isolates were studied to identify CRE where an isolate which was resistant to imipenem, meropenem, doripenem or ertapenem, was considered as CRE [3]. These isolates were further characterised with respect to age and sex of patients, site of infection (e.g., respiratory tract infections, bloodstream infections, urinary tract infections, etc.,) and healthcare settings from where they have been isolated (e.g., outpatient department, trauma and emergency, wards, Intensive care units, and autopsy room).
Prevalence of CRE was determined as number of CRE isolated per 100 Enterobacteriaceae isolates during the study period whereas incidence rate was determined as number of CRE cases per 1000 patient-days [6,7]. Prevalence with respect to carbapenem resistance of individual member of Enterobacteriaceae was also determined.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel. Defined Daily Dose (DDD) of imipenem, meropenem, doripenem and ertapenem were calculated for the period from 2014 to 2018. Yearly consumption of the carbapenems were calculated as DDD per 1000 patient-days [8].
Results
A total of 33,350 culture samples were received during the study period with a culture positivity of 11,010 (33%) including both gram positive and gram negative organisms. Out of 11,010 isolates, 4,928 (44.7%) isolates belonged to the family Enterobacteriaceae of which 1,433 (29%) isolates were resistant to atleast one of the Carbapenems (CRE). Of these, 917 (64%) were males and 516 (36%) were females. [Table/Fig-1] describes common clinical specimens from which CRE were isolated.
Specimen wise distribution of CRE isolates.
| Specimen | Number of isolates (%) |
|---|
| Urine | 416 (29%) |
| Skin and soft tissue | 330 (23%) |
| Blood | 273 (19%) |
| Sputum | 172 (12%) |
| Tracheal aspirate | 157 (11%) |
| Others | 85 (6%) |
‘Others’ include CSF, Pleural fluid, Peritoneal fluid, Drain fluid, Broncho-alveolar lavage, Stool and Vaginal swab; n=1,433
Majority of CRE isolates i.e., 702 (49%) were from critical care units, followed by 544 (38%) from wards, 86 (6%) from trauma and emergency centre, 86 (6%) from outpatient departments and 15 (1%) from post mortem samples (blood, spleenic swab, wound swab). Length of hospital stay ranged from 1 day to 94 days with an average stay of 21 days.
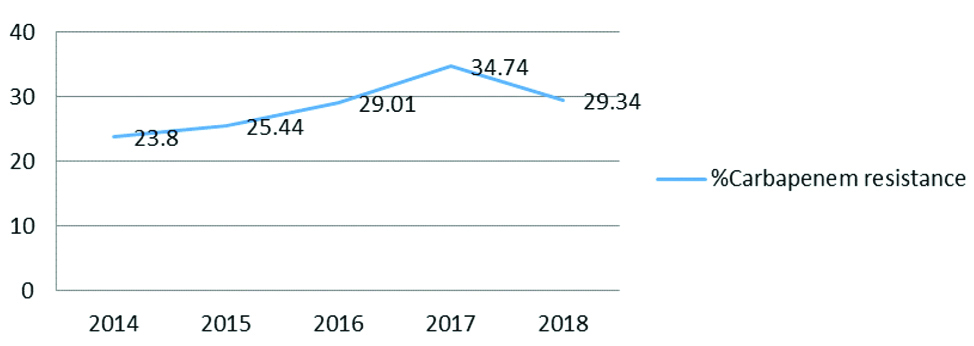
The incidence of CRE cases per 1000 patient-days in 2014 to 2018 was 1.66, 2.11, 1.90, 2.26 and 1.91, respectively with an overall incidence of 1.99 per 1000 patient-days. [Table/Fig-2] shows trend of prevalence of CRE isolated from 2014 to 2018 while [Table/Fig-3] shows distribution of Enterobacteriaceae isolates with Carbapenem resistance.
Prevalence rate of carbapenem resistance among Enterobacteriaceae from 2014-18.
Distribution of CRE isolates.
| Organism | Number (%) |
|---|
| Klebsiella sp. | 733 (51%) |
| E.coli | 439 (31%) |
| Enterobacter sp. | 137 (9%) |
| Providencia sp. | 37 (3%) |
| Proteus sp. | 35 (2%) |
| Serratia sp. | 19 (1%) |
| Citrobacter sp. | 13 (1%) |
| Morganella sp. | 12 (1%) |
| Pantoea sp. | 8 (1%) |
The overall resistance to imipenem increased from 22.95% in 2014 to 32.92% in 2017 and was reduced to 27.28% in 2018, which for meropenem increased from 22.95% in 2014 to 33.18% in 2017 and reduced to 27.36% in 2018 and for Ertapenem it increased from 23.23% in 2014 to 31.63% in 2017 and reduced to 27.88% in 2018.
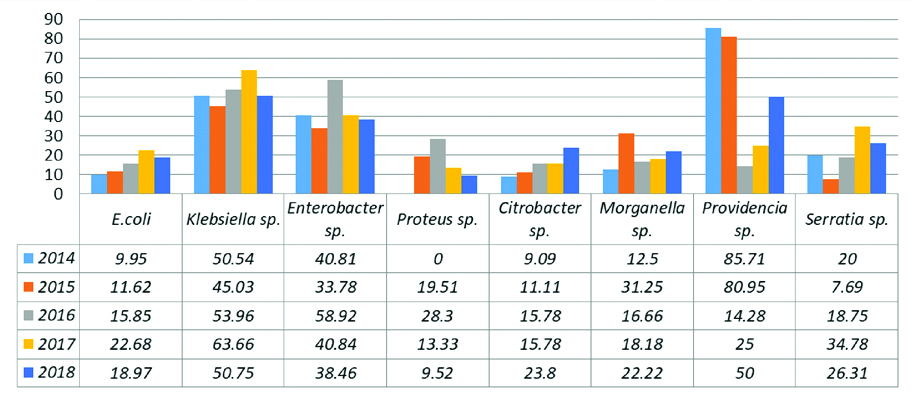
[Table/Fig-4] shows resistance to imipenem, meropenem and ertapenem among Klebsiella sp., E.coli, Enterobacter sp. and Proteus sp. during 2014 to 2018. Isolates like Providencia sp., Morganella sp., Pantoea sp. and Serratia sp. were not included as their numbers were very low.
Year-wise carbapenem resistance among Enterobacteriaceae.
| Enterobacteriaceae | Carbepenem | 2014 % Resistance | 2015 % Resistance | 2016 % Resistance | 2017 % Resistance | 2018 % Resistance |
|---|
| Klebsiella sp. | Imipenem | 49.45 | 44.27 | 53.58 | 56.9 | 36.66 |
| Meropenem | 50 | 44.65 | 52.83 | 60.9 | 49.39 |
| Ertapenem | 50 | 43.12 | 52.45 | 62.42 | 50.3 |
| E.coli | Imipenem | 8.95 | 10.52 | 14.98 | 19.33 | 17.33 |
| Meropenem | 8.7 | 10.96 | 15.15 | 21.98 | 16.85 |
| Ertapenem | 9.95 | 10.52 | 15.5 | 19.5 | 18.13 |
| Enterobacter sp. | Imipenem | 40.81 | 33.78 | 58.92 | 37.5 | 31.64 |
| Meropenem | 40.81 | 33.78 | 57.14 | 41.66 | 31.64 |
| Ertapenem | 40.81 | 32.43 | 51.78 | 37.5 | 31.64 |
| Proteus sp. | Imipenem | 0 | 43.9 | 77.35 | 73.91 | 64.06 |
| Meropenem | 0 | 19.5 | 28.3 | 13.04 | 9.37 |
| Ertapenem | 0 | 14.63 | 26.41 | 10.86 | 7.81 |
[Table/Fig-5] describes the increase in percentage of isolates with carbapenem resistance in various members of Enterobacteriaceae over a period of five years.
Percentage increase in CRE isolates.
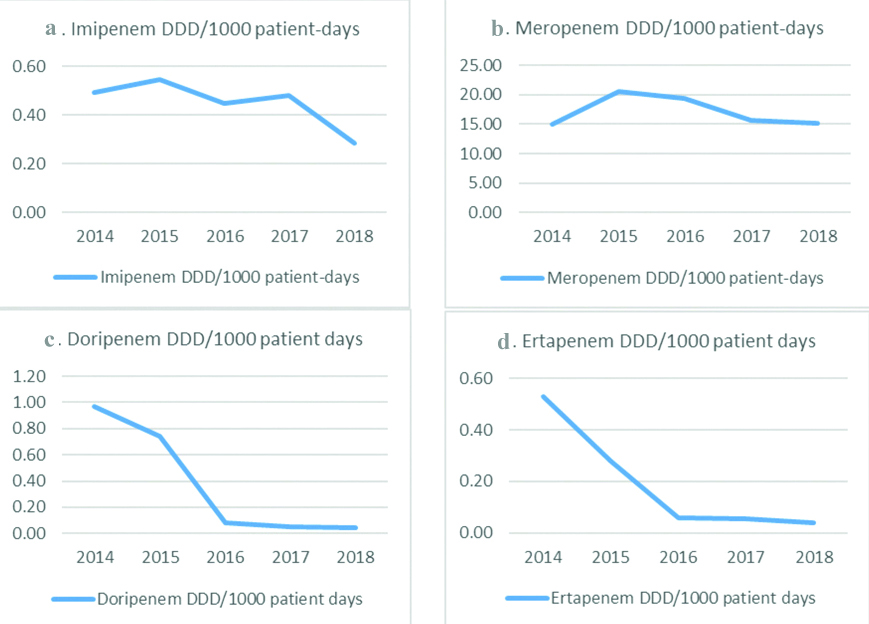
[Table/Fig-6a-d] describes yearly consumption of Carbapenems as DDD per 1000 patient-days over a period of five years. As seen in [Table/Fig-6b,d], meropenem was the most consumed carbapenem agent whereas ertapenem was the least consumed carbapenem. The average consumption of five years for meropenem was 16.95 DDD/1000 patient-days, Imipenem was 0.43 DDD/1000 patient-days, doripenem was 0.28 DDD/1000 patient-days and ertapenem was 0.15 DDD/1000 patient-days.
Yearly consumption of carbapenems.
Discussion
Carbapenems, owing to its broad antimicrobial spectrum, have been used extensively, especially for treatment of nosocomial infections [9,10]. Their use for treatment of infections caused by members of Enterobacteriaceae family has increased as the resistance of later to extended spectrum cephalosporins surfaced during 1990s [2,10]. Since then there has been an increased reporting and geographical spread of carbapenem resistance among Enterobacteriaceae with a mortality rate as high as 30-75% [1,5]. The present study was conducted to know the prevalence of CRE. The overall prevalence of CRE over a period of five years was found to be 29.07%. Moreover isolates like Providencia sp., Proteus sp. and Morganella sp. have higher baseline Minimum Inhibitory Concentrations (MICs) which might not fit into the definition of CRE adopted by CDC [2]. On excluding these isolates, the overall prevalence of CRE was 27.3% which was still higher compared to previous studies as shown in [Table/Fig-7] [1,7,11-15].
Comparison of CRE prevalence in different studies [1,7,11-15].
| Author (Publication year) | Place of study | CRE prevalence rate |
|---|
| Datta P et al., [11] (2012) | Northern India | 7.87% |
| Watkins RR and Bonomo RA [1] (2013) | Global data | 4% |
| Nair PK and Vaz MS [12] (2013) | Western India | 12.26% |
| Jan R et al., [7] (2016) | Southern India | 8% |
| Rao A and Indumathi VA [13] (2016) | Southern India | 13.95% |
| Khare V et al., [14] (2017) | Northern India | 37.9% |
| Pawar SK et al., [15] (2018) | Western India | 31.77% |
| Present study (2021) | Western India | 27.3% |
Considering the density of population in Indian scenario and the ability of the organisms to disseminate through the intestinal flora of healthy carriers as well as lack of public health infrastructure in developing countries pose a higher risk of transmission in the population leading to a higher prevalence rate. The prevalence rate of CRE in the present study increased from 23.8% in 2014 to 34.74% in 2017 which is a matter of concern and demands an urgent need of approaches that curtail proliferation of CRE in the institute. One of the major reasons for carbapenem resistance is production of chromosomally mediated or plasmid mediated enzymes i.e., Carbapenemases which are responsible for cleaving carbapenems and rendering them ineffective [16]. Plasmids are mobile genetic elements present in bacteria and can be transferred from one bacterium to another resulting in transfer of drug resistance [1,16]. Overuse of antimicrobial agents in healthcare settings lead to selection pressure for resistant strains whereas lack of early identification of infection with CRE and timely isolation of patient, and poor infection control practices promote its spread [1]. Klebsiella sp. (51%) followed by E.coli (31%) were found to be the most common isolates among CRE in the present study which is similar to other studies where Klebsiella sp. accounts for 33-46% among all CRE [9,11,12].
Overall imipenem resistance among CRE in the present study ranged from 22.9-32.9% whereas meropenem resistance ranged from 22.9-33.1% during the five year study period. Meta-analysis of data from Asian countries demonstrated imipenem resistance varying from 0.1-5.8% and meropenem resistance varying from 0.9-2.9%. Meropenem resistance from India was found to be 2.6% in one of the study [9].
[Table/Fig-4] shows year-wise carbapenem resistance in members of Enterobacteriaceae. There has been a gradual increase in carbapenem resistance in Klebsiella sp., Enterobacter sp. and Proteus sp. whereas E.coli had shown steep increase in carbapenem resistance over a period of five years. A previous study showed imipenem resistance to be 4.3% and 2.1% in Klebsiella sp. and E.coli respectively whereas meropenem resistance was found to be 6.9% and 3.5% in Klebsiella sp. and E.coli, respectively [17]. In another study, resistance to meropenem was found to be 51% and 13% whereas ertapenem resistance was found to be 61% and 20%, respectively in Klebsiella sp. and E.coli [18]. In a 12 year study, on CRE from Asian countries resistance of imipenem and meropenem in Klebsiella sp. ranged from 0.5-1.9% and 0.3-2.4%, respectively from 2000 to 2012 where as in E.coli it was found to be 0.2% and 0.1-0.5%, respectively [8]. Data from European Centre for Disease Prevention and Control (ECDC) showed carbapenem resistance up to 62% in Klebsiella sp. and 1.2% in E.coli. As per WHO Global report on antimicrobial resistance surveillance, two regions from 71 World Health Organisation (WHO) member states reported carbapenem resistance in Klebsiella sp. in excess of 50% [19].
These findings on CRE in the present study are highly significant and worrisome. Infections with CRE are difficult to treat as well as the treatment options are limited and costly, imposing additional financial burden on the patients [9]. Screening of patients for presence of CRE on admission, itself has issues of feasibility and cost. Moreover, patients with CRE colonisation or infection will require isolation facility for their management in order to prevent the spread of the drug resistant pathogen to non-infected patients. Making isolation beds and human resources available for management of these patients would be another challenge for healthcare facilities. Under these circumstances it becomes important to curb the rise of CRE by formulating and implementing antimicrobial policy that is based on the local antibiogram as well as ensure adherence to infection control practices [10]. As shown in [Table/Fig-6], there has been a reduction in consumption of carbapenems over a period of five years with implementation of antimicrobial policy. Although the prevalence of CRE has shown reduction in 2018, it still needs to be seen whether this trend persists over next few years. Klebsiella sp. and E.coli accounts for a large number of infections, both in community and hospital [5], and with such a high prevalence of carbapenem resistance amongst these isolates and their ability to spread through feco-oral route, could further aggravate the scenario in absence of robust prevention strategies. It has been well-documented that interventions such as contact isolation of patients, cohorting of colonised patients, hand hygiene, surface decontamination, routine rectal surveillance for screening of CRE and presence of antibiotic stewardship program have been effective in bringing down the resistance rates [1,5,20]. Simulation models have also shown that lack of any interventions in restricting transmission of CRE may lead to its endemicity and therefore interventions are required at local levels and even more at regional levels to slow its spread [21,22]. These interventions may seem to be a costly affair but it would be more costly and less effective once the CRE becomes endemic [5]. At our institute infection prevention and control protocols are in place, however, such a high prevalence of CRE reflects lack of proper implementation of these protocols. Authors recommend development of strategies for early identification of CRE colonised patients through active screening of high risk patients on admission and a systematic and coordinated approach towards infection prevention and control by ensuring its stringent implementation across the institute in order to confine the spread of CRE.
Limitation(s)
As the present study was a retrospective study, it was not possible for us to identify whether the CRE isolates from patients admitted in wards had been originally acquired from their stay in intensive care units or not. Similarly, it was not possible for us to identify the source of CRE isolates from patients admitted in accident and emergency. Moreover, due to lack of appropriate diagnostic resources, authors were not able to identify the mechanism of carbapenem resistance (production of carbapenemase enzymes versus other mechanisms).
Conclusion(s)
Prevalence of CRE is high in this institute. In view of limited and costly treatment options and high morbidity and mortality associated with CRE, it is necessary to control the spread of CRE. Periodic feedback on CRE rates needs to be provided to the clinical departments. Early identification and isolation of patients with stringent following of infection prevention and control practices along with judicious use of carbapenem agents would be vital in reducing the rates of CRE.
‘Others’ include CSF, Pleural fluid, Peritoneal fluid, Drain fluid, Broncho-alveolar lavage, Stool and Vaginal swab; n=1,433