The MS is an uncommon tumour composed of immature myeloid blasts with tissue destruction and poses diagnostic challenge if it is present as de novo. It may also arise in a background of AML, Myelodysplastic Syndrome (MDS), MPN and mixed MDS/MPN [1]. However, MS can occur months or even years before the development of AML [2]. With a mean interval of ten months, 27% of de novo cases of MS has been reported since discovery of isolated cases and involvement of bone marrow [3]. The diagnosis in de novo cases of MS is equivalent to the diagnosis of AML. Even in a known case of AML in remission, MS can occur as the first manifestation of relapse. It can mimic other entities histologically, clinically, and radiographically [1,2,4]. Grossly, the tumour is often described as having a green hue, earning its name “chloroma”. By conventional histopathology, it can mimic a high-grade lymphoma or other non lymphomatous tumour [1]. The application of immunomarkers is essential to distinguish MS from its mimickers. The present study was aimed to analyse MS according to different age groups, sites of involvement, evaluation of IHC and IPT markers, cytogenetics findings. The objective of the study was to discuss the differential diagnoses with the help of IHC/IPT in problematic cases to prevent misdiagnosis. Unusual sites of presentation and the pattern of expression of a substantial number of IHC/IPT biomarkers has also been evaluated by MS.
Materials and Methods
The present study was a cross-sectional retrospective study conducted in the Department of Oncopathology, in a tertiary care cancer center, The Gujarat Cancer Research Institute, Ahmedabad, India, from January, 2017 to December, 2019 with approval from the Institutional Ethical committee (No. IRC/2020/P-132). A total of 38 cases were diagnosed histologically as myeloid sarcoma and confirmed by IHC. Those cases were included in the study. Other histological mimickers were excluded. Clinical, morphological, IHC, IPT and molecular data of 38 myeloid sarcoma cases were retrieved from pathology archives.
Collection and preparation of samples: The peripheral blood and bone marrow material were collected in Ethylenediaminetetraacetic Acid (EDTA) containing vacutainer for microscopic smear examination and IPT. A microscopic evaluation was done using Wright-stained peripheral smears and bone marrow aspirates. All the samples were processed within 24 hours. The primary antibody panel composed of the following monoclonal antibodies: CD45 (PerCP), CD22 (FITC), CD34 (PE), CD7 (FITC), CD13 (PE), CD33 (PE Cy7), CD117 (APC), HLA-DR (APC-H7), MPO (FITC), CD5 (PE Cy7), CD10 (APC), CD19 (APC-H7), cCD79a (PE), cCD3 (PE Cy7), and Tdt (APC) whereas the secondary panel included CD2 (FITC), CD4 (PE Cy7), CD8 (APC-H7), CD11b (PE Cy7), CD11c (PE), CD14 (APC-H7), CD15 (FITC), CD1a (PE), CD41a (PE), CD41b (FITC), and CD61 (FITC). For blast gating, the CD45 was used for both cell surface and cytoplasmic markers. BD Fluorescence-Activated Cell Sorting (BD FACS) Lysing solution was used to lyse RBC and BD perm wash buffer was used to permeabilised cells for intracellular markers. All reagents were obtained from BD Biosciences, USA.
Immunophenotyping using flowcytometry: For the surface monoclonal antibodies, respective antibody (20 μL) mentioned above was put in six-colour combination to the peripheral blood or bone marrow sample (100 μL, 1×10). After incubation for 15 minutes, 2 mL of erythrocyte lysing solution (1:10 dilution with double distilled water; BD Biosciences, USA) was added and again incubated (15 min; room temperature). The cell centrifugation was done at 400 g for five minutes. The supernatant was discarded. The remaining pellet was washed twice with Phosphate-Buffered Solutions (PBS) and resuspended in 500 μL of PBS.
For the cytoplasmic antibodies, first to lyse the RBCs in peripheral blood or bone marrow, 2 mL lysing solution was given to 100 μL of sample and incubated for 15 minutes and centrifuged. After centrifugation, buffer (1 mL per wash) was added to permeabilise the cells, incubated (20 min) and centrifuged. The respective antibody (20 μL) was added to the pellet and incubated (15 min). After that, 2 mL PBS was added and the samples were centrifuged (1500 rpm×5 min). The supernatant was discarded. The pellet was resuspended in 500 μL of PBS. Negative control tubes were run simultaneously for cell surface and cytoplasmic markers with the sample and CD45 antibody.
Data analysis: For daily calibration, the cytometry setup and tracking beads were (BD Biosciences, USA) used. The samples were run in Flow cytometer (FACS Canto II, 6-colour, 2-Laser, BD Biosciences, San Jose, CA 95131, USA) and analysed (FACS Diva software, BD Biosciences, San Jose, CA 95131, USA). A minimum of 100000 total cells were acquired. For blast gating, the side scatter versus CD45 PerCP dot plot was used. A positive result indicated a cut off value of more than 20% for the surface or intracellular markers according to the principles of flowcytometry (BD Biosciences, USA).
Immunohistochemistry (IHC): IHC was performed by fully automated machine, Ventana XT Benchmark at the time of initial presentation on formalin-fixed, paraffin-embedded, four to 5-micronsections using monoclonal antibodies to MPO (Thermo Scientific-1:50, Ab1), CD117 (Cell Marque-1:100, YR145), CD68 (Cell Marque-1:100, KP1), CD34 (DAKO-1:50, QBEnd10), CD45 (Thermo Scientific-1:100, RA/RO), CD43 (DAKO-1:50, DF-T1), CD99 (Thermo Scientific-1:50, H036-1.1), TdT (BioGenex-1:30, EP266), Vimentin (BioGenex-1:200, B9), Desmin (BioGenex-1:30, D33), Friend Leukaemia Integration-1 (FLI-1) (Cell Marque-1:75, MRQ-1), B-cell lymphoma 2 (Bcl2) (Cell Marque-1:100, 124), B-cell lymphoma 6 (Bcl6) (Cell Marque-1:100, GI191E/A8), Multiple Myeloma1 (MUM1) (Cell Marque-1:100,MRQ43), CD2 (Cell Marque-1:100, MRQ11), CD3 (DAKO-1:50, F7.2.38), CD10 (Ventana-RTU, SP67), CD20 (Cell Marque-1:100, L26), CD79a (Cell Marque-1:100, JCB117), CD1a (BioGenex-1:30, O10), CD15 (DAKO-1:50, Carb-3), CD13 (Thermo Scientific-1:50,38C12) Epithelial Membrane Antigen (EMA) (Cell Marque-1:100, E29), Anion Exchanger1/3(AE1/AE3) (Thermo Scientific-1:100, AE1/AE3), Inhibin alpha (Cell Marque-1:100,R1), CD19 (Cell Marque-1:100, MRQ36), Synaptophysin (Thermo Scientific-1:50, SP11), Chromogranin A (Cell Marque-1:100, LKH110) and Glial Fibrillary Acidic Protein (GFAP) (BiGenex-1:30, GA-5). Minimum and maximum number of markers applied was three and 12, respectively.
Cytogenetics studies were performed in the institute in all 16 AML cases to look for t(8;21) as well as inversion of long arm of chromosome 16 {inv(16)} and all suspected 13 cases of CML to confirm the presence of t(9;22).
Statistical Analysis
Mean and median were calculated. Data were analysed by Microsoft Office Excel Datasheet 2019.
Results
In this study, total 38 patients were registered with an age range from five years to 68 years. Mean and median ages were 31.13 years and 32 years respectively. Of them, nine patients were under 18 years with a male-female ratio of 2:1 and 29 patients having more than 18 years of age with a male-female ratio of 3.8:1 [Table/Fig-1]. Out of 38 cases, 16 (42.1%) and 13 (34.2%) cases were previously diagnosed cases of AML and CML respectively. Nine patients (23.7%) presented de novo without a prior diagnosis of myeloid leukaemia.
Distribution of patients according to gender and age.
| Sex | <18 yearsn (%) | >18 yearsn (%) | Totaln (%) |
|---|
| Male | 6 (15.8) | 23 (60.5) | 29 (76.3) |
| Female | 3 (7.9) | 6 (15.8) | 9 (23.7) |
| Total N (%) | 9 (23.7) | 29 (76.3) | 38 (100) |
In present study, the most common site of MS was lymph node (12/28, 31%) followed by breast, vertebra, and other uncommon sites including thalamus, thyroid, prostate, uterus, cervix and ileum [Table/Fig-2]. Expression of myeloid and other biomarkers by IPT as well as IHC was analysed. According to the sites, in de novo cases, differential diagnoses were considered and immunomarkers were applied accordingly.
Distribution of patients according to site (N=38).
| Location | Number of cases n (%) |
|---|
| Anterior mediastinum | 1 (2.6) |
| Breast | 3 (7.7) |
| Cervix | 1 (2.6) |
| Chest wall | 1 (2.6) |
| Eye | 2 (5) |
| Gluteal region | 1 (2.6) |
| Hip joint | 1 (2.6) |
| Ileum | 1 (2.6) |
| Leg | 2 (5) |
| Lymph node | 12 (31) |
| Nasal | 1 (2.6) |
| Orbit | 2 (5) |
| Perianal | 1 (2.6) |
| Prostate | 1 (2.6) |
| Scapula | 1 (2.6) |
| Submandibular gland | 1 (2.6) |
| Thalamus | 1 (2.6) |
| Thyroid | 1 (2.6) |
| Tibia | 1 (2.6) |
| Uterus | 1 (2.6) |
| Vertebra | 2 (5) |
Myeloid Sarcoma (MS) in known AML patients: Total of 16 patients were registered in this group. Mean time interval from diagnosis of AML and the development of MS was five months. Tumours expressed myeloid markers either by IPT flowcytometry or by IHC. MPO, CD117, CD45, CD43, CD68, CD13, CD33, CD34 and HLA-DR showed consistent positivity [Table/Fig-3,4]. Two cases with strong morphological suspicion of monocytic lineages showed CD14 and CD64 expression. Aberrant CD7 expression was present in IPT of one patient [Table/Fig-5]. One patient, 14-year male, having mass in anterior mediastinum also had myeloid blast cells in pleural fluid [Table/Fig-5,6]. But none of them expressed mature lymphoid, epithelial or other mesenchymal markers. On cytogenetic analysis, one of the patients had t(8;21) and two other patients had an inversion of the long arm of chromosome 16 (Inv16).
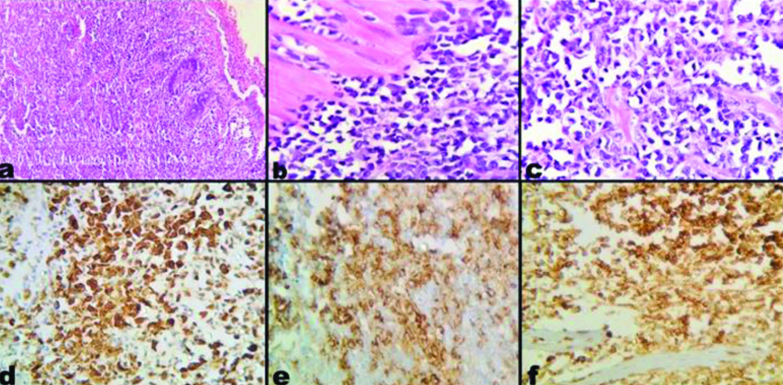
Myeloid Sarcoma (MS) a) Low power view showing intestinal epithelium with diffuse infiltration by neoplastic cells into the lamina propria and deeper layers. (H&E, 10x); b) High power view showing medium to large myeloid blasts infiltrating through the muscularis propria (H&E, 40x); c) Myeloid blasts with irregular nuclear contour, fine chromatin, small nucleoli and moderate amount of cytoplasm (H&E,40x); d) Staining with MPO (IHC,40x); e) Staining with CD34 (IHC,40x); f) Staining with CD45 (IHC,40x).
H&E: Hematoxylin and Eosin; IHC: Immunohistochemistry; MPO: Myeloperoxidase
IHC/IPT marker expression in myeloid sarcoma.
| Immunomarkers | AML (n=16) IHC/IPT (%) | CML (n=13) IHC/IPT (%) | De novo (n=9) IHC/IPT (%) | Total (n=38) |
|---|
| MPO | 13/16 (81.2) | 9/13 (69.2) | 9/9 (100) | 31/38 (81.5) |
| CD117 | 11/15 (73.3) | 7/12 (58.3) | 7/8 (87.5) | 25/35 (71.4) |
| CD34 | 8/16 (50) | 4/12 (33.3) | 3/9 (33.3) | 15/37 (40.5) |
| CD68 | 4/12 (33.3) | 4/12 (33.3) | 4/4 (100) | 12/28 (42.8) |
| CD45 | 8/9 (88.8) | 4/5 (80) | 9/9 (100) | 21/23 (91.3) |
| CD43 | 5/9 (55.5) | 5/8 (62.5) | 4/5 (80) | 14/22 (63.6) |
| CD99 | 2/10 (20) | 1/10 (10) | 0/9 (0) | 3/29 (10.3) |
| CD13 | 8/9 (88.8) | 4/4 (100) | 3/3 (100) | 15/16 (93.7) |
| CD33 | 9/9 (100) | 4/4 (100) | 1/1 (100) | 14/14 (100) |
| HLA-DR | 7/8 (87.5) | 3/3 (100) | 1/1 (100) | 11/12 (91.6) |
| CD14 | 2/2 (100) | 0/0 | 0/0 | 2/2 (100) |
| CD64 | 2/2 (100) | 0/0 | 0/0 | 2/2 (100) |
| CD7 | 1/16 (6.2) | 1/13 (7.6) | 0/0 | 1/29 (3.4) |
| Vimentin | 0/3 | 0/0 | 0/9 | 0/12 |
| FLI1 | 0/3 | 0/0 | 0/4 | 0/7 |
| TdT | 1/7 (14.2) | 0/2 | 0/0 | 1/9 (11.1) |
| CD2/CD3/CD10/CD20/MUM1 | 0/16 | 0/13 | 0/4 | 0/33 |
| CD1a/Bcl2/Bcl6/CD138/CD79a | 0/2 | 0/2 | 0/4 | 0/8 |
| AE1/EMA/Inhibin/Desmin | 0/3 | 0/2 | 0/1 | 0/5 |
| GFAP/SNP/CGA | 0/0 | 0/0 | 0/1 | 0/1 |
CD: Cluster of differentiation; CGA: Chromogranin A; EMA: Epithelial membrane antigen; GFAP: Glial fibrillary acidic protein; HLA: Human leucocyte antigen; SNP: Synaptophysin; TdT: Terminal deoxynucleotidyl transferase
a) Flowcytometry image showing myeloid blasts expressing CD34, CD117, MPO, CD13. CD33, CD15 and HLA-DR with aberrant expression of CD7; b) Smear prepared from pleural fluid of the same patient shows infiltration by myeloid blasts having round to oval convoluted nuclei, fine chromatin, small nucleoli and moderate to abundant cytoplasm (Papanicolaou stain, 40x); HLA- Human leukocyte antigen; MPO- Myeloperoxidase.
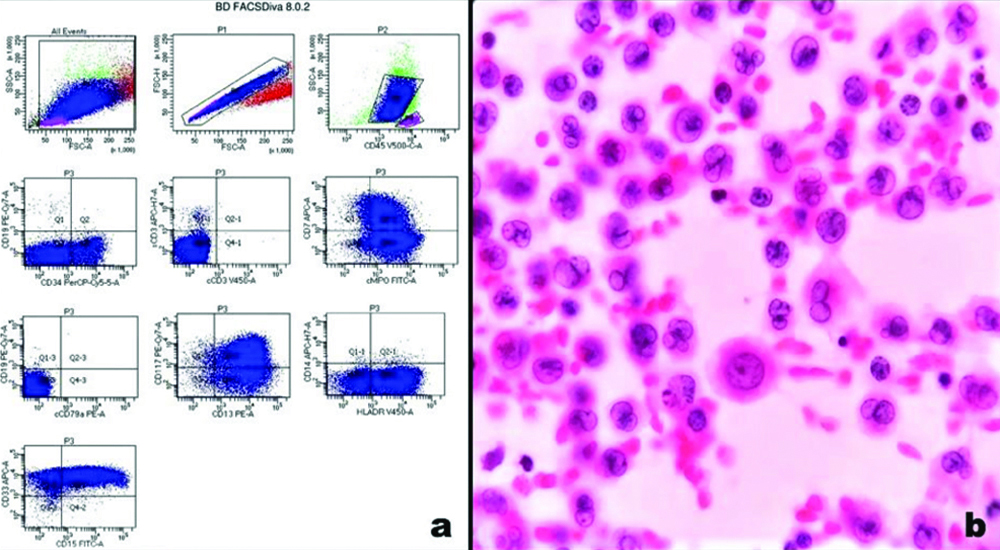
Distribution of cases according to CSF/Pleural fluid cytology.
| Variables | AML | CML | De novo |
|---|
| P | N | ND | T | P | N | ND | T | P | N | ND | T |
|---|
| CSF (n) | 0 | 12 | 14 | 16 | 2 | 9 | 2 | 13 | 1 | 7 | 1 | 9 |
| Pleural fluid (n) | 1 | 0 | 15 | 16 | 1 | 0 | 12 | 13 | 0 | 0 | 9 | 9 |
P: Positive; N: Negative; ND: Not done; T: Total
Myeloid Sarcoma (MS) in known CML patients: There were total of 13 registered cases in this group and all of them presented with CML blast crisis phase in marrow or peripheral blood. Mean time interval from the initial diagnosis of CML and development of MS was two years. All the cases were confirmed by t(9;22) cytogenetics at initial work up. Out of 13 cases, two cases were variant positive for t(9;22){t(2;9;22) p23;q34;q11.2}. Myeloid markers were expressed. One case expressed CD99 along with myeloid markers and another one case showed aberrant CD7 expression in IPT. Epithelial, mesenchymal and mature lymphoid markers were negative in all cases [Table/Fig-4]. CSF and pleural fluid were involved by myeloid blast cells in two (one adult, one paediatric) and one patient (adult), respectively [Table/Fig-6].
Myeloid Sarcoma (MS) in de novo cases: Total of nine cases were registered in this study. They all presented with mass in different sites of the body without any evidence of increased blast cells either in marrow or peripheral blood smear. Involved sites were breast, leg, thalamus, eye, vertebra, ileum and ovary with uterus. Mean bone marrow blast count was 2.8% (Range 1-5%) at initial presentation. Differential diagnoses were considered and IHC was performed accordingly.
Discussion
MS is an extramedullary mass composed of neoplastic immature myeloid blasts with tissue destruction. It is usually a straightforward diagnosis in a known patient of AML. However, it becomes really challenging when it presents de novo at an unusual site. In that scenario, clinicopathological features including the support of IHC/IPT is of utmost importance.
In several published studies, myeloid sarcoma affected male predominantly, supporting present study finding (Male:Female- 3:1) [1,5]. The age range of the patients at first presentation is highly variable. Mean age in present study was 31.1 years, similar to a previous study by Byrd JC et al., [6]. In present study, the commonest site of involvement was lymph node (31%). This is corroborative with other studies, where they found reticuloendothelial system as the most commonly affected [3,5]. In the literature, histopathology proven MS is mostly reported in the skin, bone and lymph nodes. However, many other sites can be involved like the central nervous system, retroperitoneum, oral cavity, mucosa of nose, breast, urinary tract, chest wall, pleura, gastrointestinal tract and testis, similar to present findings [1,3,7-12]. In children, skin (54%) was the most common extramedullary involvement followed by orbit with newly diagnosed AML [13]. In present study, extramedullary involvement in diagnosed AML patients under 18 years of age was mainly in orbit, skin of leg and anterior mediastinum.
Over the time, MS has been subdivided into two categories, either granulocytic or monoblastic, based on morphology [2]. However, this division is of less relevance in context of cytogenetics. Also, IPT has proved that MS can express multiple lineages within a same tumour [2]. Classic histomorphology of MS show effacement of tissue architecture by myeloblasts with round to oval nuclei, fine nuclear chromatin, small nucleoli and scant cytoplasm. Such features in a tumour at unusual site should raise an index of suspicion for MS. Initial diagnosis of MS is straightforward with classic histology and immunoreactivity. However, at initial presentation, deceptive histomorphology along with the absence of any history of AML/MPN may lead the diagnosis very challenging.
Evaluation of IHC/IPT markers: Many studies have been published on the expression of immunomarkers by MS [1,5,14-19]. The results of present study have been compared with the published series [Table/Fig-7] [1,2,14,16,17,19]. MPO and CD68 antigen are frequently expressed [20]. Present study found MPO and CD68 expressions in 81.5% and 42.8% of the cases respectively. CD34 expression was seen in 40.5% of present cases, which is an inconsistent finding [21]. In a large study, CD34 expression was present in 40/92 (43.4%) of the cases while it is frequently negative in tumours with monocytic differentiation [1,21]. However, in a study, CD34 was found to be expressed in 100% of the cases [5]. Chang CC et al., in a study, showed HLA-DR to be more sensitive than CD34 for MS [17]. We also found much higher (91.6%) HLA-DR expression in comparison to CD34 (40.5%). In IPT, though CD45 (leukocyte common antigen) dim population is considered as myeloid blasts, IHC is variable. In one study, only 14 out of 24 cases (58%) showed immunoreactivity for CD45 [16]. But present study found CD45 expression in 91.3% of cases. Further expression of CD33, CD13 and CD117 were found frequently i.e., 100%, 93.7% and 71.4%, respectively, similar to the other published series [18,22]. Two cases in present study showing monocytic differentiation expressed CD14 and CD64. Usefulness of IHC for CD14 in monocytic leukaemia has been well described in literature [2].
Comparison of immunomarkers in different studies [1,2,14,16,17,19].
| Immunomarker | Percent (%) positivity of immunomarkers |
|---|
| Pileri SA et al., [1] (n=92) | Al- Khateeb H et al., [5] (n=21) | Traweek ST et al., [14] (n=28) | Menasce LP et al., [16] (n=26) | Chang CC et al., [17] (n=17) | Garcia MG et al., [19] (n=11) | Present Study (n=38) |
|---|
| MPO | 83.6 | 85 | 93 | 88.4 | 87.5 | 100 | 81.5 |
| CD117 | 80.4 | 90 | NA | NA | NA | 87.5 | 71.4 |
| CD34 | 43.4 | 100 | 36 | NA | 38 | 66.6 | 40.5 |
| CD68 | 51 | 43 | 79 | NA | 53.5 | 50 | 42.8 |
| CD45 | NA | 33 | 75 | 53.8 | NA | 100 | 91.3 |
| CD43 | NA | 10 | 100 | 100 | NA | NA | 63.6 |
| CD99 | 54.3 | 15.3 | NA | 15.3 | NA | NA | 10.3 |
| CD13 | NA | NA | NA | NA | NA | NA | 93.7 |
| CD33 | NA | NA | NA | NA | NA | NA | 100 |
| HLA-DR | NA | NA | NA | NA | 85.8 | NA | 91.6 |
| CD14 | NA | NA | NA | NA | NA | NA | 100 |
| CD64 | NA | NA | NA | NA | NA | NA | 100 |
| CD7 | NA | NA | NA | NA | NA | NA | 3.4 |
| TdT | 31.5 | 5 | NA | NA | NA | NA | 11.1 |
| CD3 | N | NA | N | N | NA | N | N |
| CD20 | NA | NA | N | N | NA | N | N |
| CD10 | NA | NA | N | NA | NA | NA | N |
| CD79a | NA | NA | N | N | NA | NA | N |
N: Negative; NA: Not available
IHC Markers in Differential Diagnosis
In adult population: Non-Hodgkin Lymphoma (NHL) is the most common differential diagnosis. So, addition of B-cell markers to the IHC panel is essential as cells of MS usually don’t express CD20 and/or CD79a, although in rare cases, weak immunoreactivity of PAX-5 and CD79a associated with t(8;21) have been reported in the literature [14,16,23]. In present study, none of the cases expressed Bcl2, Bcl6, CD10, CD20, CD79a or MUM1 [Table/Fig-3].
Distinguishing MS from a mature T-cell lymphoma is very challenging as both the entities frequently express CD43 and CD45. The presence of immature eosinophilic precursors within the background inflammatory infiltrate are highly suggestive of MS. Similar to present finding of aberrant expression of CD7 in two cases, AMLs have been reported to express T-cell antigens, such as CD7 [24,25]. In those scenarios, addition of MPO and CD68 will prevent a misdiagnosis of NHL. In present study, no case was found to express T cell markers like CD2 and CD3.
Histiocytic Sarcomas (HS) at extra-nodal sites, including skin are less likely to present with very similar histologic, and IPT features with that of AML with skin involvement. In present study, two de novo cases presented with leg ulcer raising HS as a differential diagnosis. In addition to histiocytic marker CD68, CD45 and HLA-DR may be positive in HS [26-27]. But it lacks CD33 and CD13 expression.
In present study, the possibility of primary or metastatic poorly differentiated carcinoma was excluded by AE1/AE3 and EMA negativity in all suspected cases. However, rarely myeloid sarcomas can show scattered dotlike cytokeratin immunoreactivity, but expression of EMA has not been documented [1]. Lack of expression of CD99 and Inhibin ruled out sex cord stromal tumour in case of mass involving ovary, uterus and cervix. In case of the vertebra, negative CD138 and MUM1 excluded the diagnosis of plasmacytoma. In case of thalamic lesion negative immunostaining for GFAP ruled out the diagnosis of glioma.
In paediatric population: In the paediatric population, distinguishing MS from Ewing Sarcoma/Primitive Neuroectodermal Tumour (ES/PNET), or other small round cell tumours can be challenging, as a major percentage (54.3%) of MS express CD99 [28]. However, antibodies for MPO, CD117, CD68 and/or CD43 antigen are necessary to obtain a correct diagnosis in the presence of CD99 immunoreactivity.
As MS frequently express TdT, Lymphoblastic lymphoma is also a differential diagnosis [25]. In a study by Kang LC and Dunphy CH, co-expression of TdT and CD99 were reported in 11/49 cases (22.4%) of AML [29]. In present study, we found one case (11.1%) expressing nuclear TdT along with other myeloid markers.
Lack of expression of Vimentin and Desmin ruled out possibility of rhabdomyosarcoma in one of the patients.
Cytogenetics: In present study, among the 16 known patients of AML, one male patient, nine-year-old, who presented with mass in orbital region, showed t (8;21) and a 25-year-old female who had a mass in ileum showed inv (16) in the cytogenetic study. In a study of conventional cytogenetics, trisomy 8 and inv (16) (p13.1q22) were reported as the most frequent abnormalities [18]. As reported in the literature, the site of MS and age of presentation have an association with the cytogenetics. MS with t(8;21) (q22;q22) are usually found in the orbital region in children, whereas those with inv(16) (p13.1q22) are frequently found in the gastrointestinal tract or breast in adult population [1,18,30]. These published studies support present findings. Apart from the cases of CML having t(9;22), two cases were variant positive for t(9;22) {t(2;9;22) p23;q34;q11.2}. A Cytogenetic study from the tumour tissue in de novo cases was not done.
Limitation(s)
The study is limited by the small sample size within a short time period.
Conclusion(s)
The present study represents a comprehensive analysis of myeloid sarcoma in Indian population according to the age, gender, site of involvement, differential diagnoses, IHC, IPT and cytogenetics. Raising high index of suspicion especially in de novo cases and appropriate use of immunomarkers help a pathologist to make a correct diagnosis of MS at unusual sites.
CD: Cluster of differentiation; CGA: Chromogranin A; EMA: Epithelial membrane antigen; GFAP: Glial fibrillary acidic protein; HLA: Human leucocyte antigen; SNP: Synaptophysin; TdT: Terminal deoxynucleotidyl transferase
P: Positive; N: Negative; ND: Not done; T: Total
N: Negative; NA: Not available