Introduction
Bladder cancer is the most common malignancy involving the urinary system and the ninth most common malignancy worldwide. Ki-67 is a nonhistone cellular marker for proliferation. HER2/neu is an oncogene that plays an important role in the pathogenesis of many cancer types. In bladder carcinoma, its clinical significance remains under-investigated and poorly linked to the patients’ clinicopathological features especially with no reported Egyptian study.
Aim
The aim of this work was to study the expression of HER2/neu and Ki-67 in urinary bladder carcinoma to evaluate their role in tumourigenesis and their correlation with other available clinicopathological variables associated with urothelial carcinoma.
Materials and Methods
This cross-sectional study was conducted at the Department of Pathology, Faculty of Medicine, Cairo University, Egypt. Samples were paraffin blocks from 60 cases diagnosed with urothelial carcinoma underwent radical cystectomy. Ki-67 and HER2/neu immunohistochemical staining was done and of Ki-67 and HER2/neu Immunostaining was recorded. The associations between Ki-67, HER2/neu expressions and clinical and histopathological parameters of urothelial bladder carcinoma was evaluated.
Results
The Ki-67 expression had significant association with tumour histological grade and lymphovascular invasion (p-value <0.05). The association of HER2/neu expression had significant association with perineural invasion (p-value <0.05).
Conclusion
HER2/neu immunostaining was not associated with most of the clinicopathologic prognostic factors in urothelial bladder carcinoma.
Bladder cancer, Cellular marker, Immunohistochemistry
Introduction
Bladder cancer is the ninth most common malignancy worldwide that is often diagnosed in older adults. It accounts for about 7% of all new cases of cancer in men [1,2]. Many occupational and environmental hazards are considered as risk factors for bladder carcinoma. In Egypt, schistosomal infection is another major risk factor because of the high prevalence of this parasite [3]. Though it is commonly referred to “bladder cancer”, bladder neoplasms represent a broad spectrum of disease, about 95% of bladder tumours are of epithelial origin [4]. In developing countries particularly in the Middle East and Africa, the majority of bladder cancers are Squamous Cell Carcinomas and most of these cancers are secondary to Schistosoma haematobium infection. Some studies from Egypt have shown a reversal of this trend due to better control of schistosomiasis in the region, whereas in other parts of Africa the association is mostly unchanged [5].
The major prognostic factors in bladder carcinoma are the depth of invasion into the bladder wall and the degree of differentiation of the tumour [6]. Ki-67 is a nonhistone nuclear protein that is encoded by the MKi-67 gene in humans and is a cellular marker for proliferation which is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent from resting cells (G0) [7].
In urothelial carcinoma, although tumour grade and stage are considered signs of aggressive behaviour for bladder cancer, several reports in the literature describe a correlation between Ki-67 labeling index with well-known prognostic factors, such as grade and stage [8]. HER2/neu is a transmembrane receptor tyrosine kinase, and its coding gene is located on chromosome band 17q21, a known proto-oncogene [9]. Its overexpression is associated with poor cancer prognosis and anti-HER2/neu therapy is well established for HER2/neu overexpressing breast cancers and gastric cancers [10,11].
In urothelial carcinomas, HER2/neu protein overexpression and gene amplification have been reported, and some studies have shown that it has a prognostic significance in them, which is an important finding needed to be investigated further. Some studies considered HER2/neu as a new therapeutic target for urothelial carcinomas [12,13]. In addition, a recent study by Kumar M found a significant correlation between immunohistochemical expression of HER2/neu and Ki-67 and the World Health Organisation (WHO) 2004 grade of urothelial carcinoma and concluded that these markers can be used to aid in assessing high grade urothelial tumours in controversial cases, in which the decision between low and high grade urothelial tumours is crucial [14]. Since no such previous report was done on Egyptian population and the previous studies in general had some controversy. So, the aim of this study was to evaluate the immunohistochemical expression of both Ki-67 and HER2/neu in urinary carcinoma and to correlate it with the different available clinicopathological data.
Materials and Methods
The cross-sectional study was conducted between January 2016 and November 2018 after receiving the Institutional Medical Ethics Committee approval. Formalin fixed paraffin blocks from 60 cases of diagnosed urothelial carcinoma who underwent radical cystectomy were collected from the archives of the Pathology Department, Faculty of Medicine, Cairo University, Egypt.
Inclusion criteria: The radical cystectomy specimens specimens diagnosed as urothelial carcinoma.
Exclusion criteria: Radical cystectomy specimens with other causes in the urinary bladder.
The clinical data of the cases were taken from their requisition sheets enclosed with the specimens. Serial sections were cut from each block and were stained with Haematoxylin & Eosin for histopathological assessment and another two sections were mounted on charged slides for immunohistochemical staining with monoclonal antibody against Ki-67 antigen clone MIB-1 and polyclonal rabbit anti-human c-erbB-2 oncoprotein (HER2/neu) respectively.
Immunohistochemical Assessment
HER-2/neu: The brown membranous staining intensity and pattern were considered for scoring according the following scheme: 0, no staining or membrane staining observed in less than 10% of the tumour cells; 1+, partial faint membrane staining in more than 10% of the tumour cells; 2+, circumferential week to moderate staining observed in more than 10% of the tumour cells; 3+, circumferential strong membrane staining observed in more than 10% of the tumour cells. Scores of 2+ and 3+ were considered positive for HER2/neu [15].
Ki-67: The percentage of positive cells (labeling index, LI) was calculated. Each slide was given a value composed of the sum of staining intensity and the proportion of the stained cells. This proportion was graded as:0 for 0-10% of tumour cells stained, 1 for 11-25% of cells stained, 2 for 26-50% of cells stained and 3 for >50% of cells stained. Staining intensity was graded as: 1 for light yellow, 2 for dark yellow and 3 for brown. The final staining Quantification value was as follows: 0 for negative (1-2), 1+ for mild (3), 2+for moderate (4), and 3+ for strong (5-6) [16].
Statistical Analysis
All collected data were revised for completeness and consistency. Pre-coded data was entered on the computer using "Microsoft Office Excel Software" program (2017) for windows. Data was transferred to the Statistical Package of Social Science Software program, version 16.0 (SPSS) to be statistically analysed. Descriptive statistics were used to describe variables: mean, and standard deviation for quantitative variables. Comparison between groups was performed using chi square test for qualitative variables. The p-value less than 0.05 were considered statistically significant.
Results
The age of the patients ranged between 28 and 77 years with a mean age of 59.57±9.39 years. Forty cases were above the age of 60 (66.67%) and 20 cases (33.33%) were below this age. Gender distribution showed that most of the studied cases were males; 54 out of 60 cases representing (90%) with a male to female ratio was 9:1 as seen in [Table/Fig-1].
Demographic data of the studied cases (N=60).
| Variable | Number | % |
|---|
| Age (Years) | <60 | 20 | 33.33 |
| ≥60 | 40 | 66.67 |
| Sex | Male | 54 | 90 |
| Female | 6 | 10 |
Distribution of the histopathologic features according to the available data (tumour size, site, gross appearance, histologic type and grade, multifocality, association with Bilharziasis, T stage, nodal status, lympho-vascular invasion and perineural invasion) among the cases is represented in [Table/Fig-2].
Distribution of the histopathologic features among the cases (N=60).
| Histopathologic features | Number (%) |
|---|
| Tumour size (greatest dimension) |
| <4 cm | 19 (32) |
| ≥4 cm | 41 (68) |
| Tumour site within the bladder |
| Dome | 14 (23.33) |
| Anterior wall | 12 (20) |
| Posterior wall | 19 (31.66) |
| Right lateral wall | 3 (5) |
| Left lateral wall | 3 (5) |
| Most of the walls or the whole cavity | 9 (15) |
| Tumour gross appearance |
| Fungating | 38 (63.33) |
| Ulcerative | 17 (28.33) |
| Infiltrative | 5 (8.33) |
| Tumour histologic type of urothelial carcinoma |
| Conventional | 50 (83.3) |
| Invasive papillary | 4 (6.6) |
| With squamoid differentiation | 4 (6.6) |
| Plasmacytoid | 1 (1.6) |
| Clear cell | 1 (1.6) |
| Histologic grade |
| Low grade | 27 (45) |
| High grade | 33 (55) |
| Association with bilharziasis |
| Associated | 32 (53.3) |
| Not associated | 28 (46.7) |
| Tumour multifocality |
| Unifocal | 11 (18.3) |
| Multifocal | 49 (81.2) |
| T stage |
| T1-T2 | 22 (36.6) |
| T3-T4 | 38 (63.3) |
| Nodal status |
| Positive | 16 (26.7) |
| Negative | 44 (73.3) |
| Lympho-vascular invasion |
| Present | 33 (55) |
| Absent | 27 (45) |
| Perineural invasion |
| Present | 22 (36.7) |
| Absent | 38 (63.3) |
Immunohistochemical expression of HER2/neu and Ki-67: Positive HER2/neu expression was seen in 38 cases (representing 63.3%) and negative in 22 cases (36.7%) [Table/Fig-3]. Out of 60 cases, 21 (35%) of the studied cases of Ki-67 expression showed strong expression, 20 cases (33.3%), 14 cases (23.3%), five cases (8.3%) showed moderate, mild and negative Ki-67 expression respectively [Table/Fig-4].
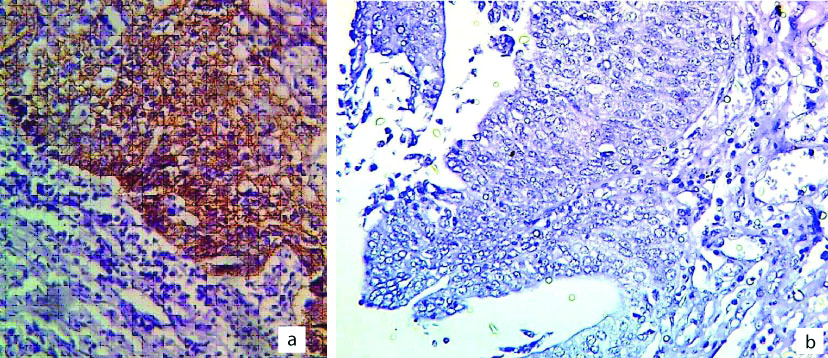
a) Positive membranous HER2/neu expression in high grade urothelial carcinoma; 400X; b) Negative membranous HER2/neu expression in high grade urothelial carcinoma; 400x.
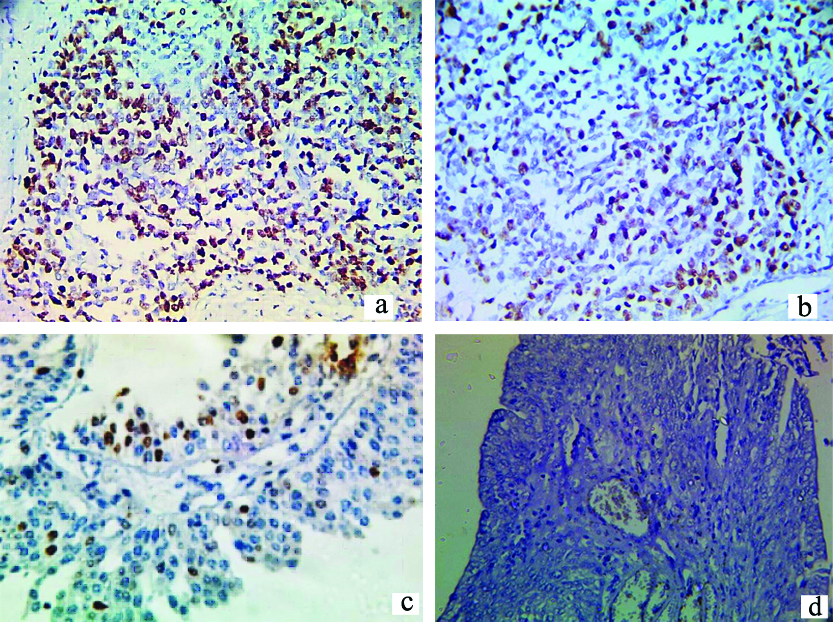
a) Strong positive nuclear Ki-67 expression in high grade urothelial carcinoma; 400x; b) Moderate positive nuclear Ki-67 expression in high grade urothelial carcinoma; 400x; c) Mild Ki-67 expression in high grade invasive papillary carcinoma; 400x; d) Negative Ki-67 expression in high grade invasive papillary carcinoma; 40x.
The relation between HER2/neu expression and most of the studied clinicopathologic features was insignificant except for the presence of perineural invasion (p=0.024) [Table/Fig-5]. The relation between Ki-67 expression and most of the studied clinicopathologic features was insignificant except for the histologic grade of the tumour (p<0.001) and the presence of lympho-vascular invasion (p=0.002) [Table/Fig-6]. The relation between Ki-67 expression and HER2/neu positivity was statistically insignificant (p-value=0.99) [Table/Fig-7].
Relation between HER2/neu immunostaining and clinicopathologic features.
| Variable | Total N (60) | HER2/neu immunostaining N (%) | p-value |
|---|
| Negative (22) | Positive (38) |
|---|
| Age (Years) | <60 | 20 | 9 (45) | 11 (55) | 0.344 |
| ≥60 | 40 | 13 (32.5) | 27 (67.5) |
| Sex | Male | 54 | 19 (35.2) | 35 (64.8) | 0.86 |
| Female | 6 | 3 (50) | 3 (50) |
| Tumour size | <4 cm | 19 | 9 (47.4) | 10 (52.6) | 0.242 |
| ≥4 cm | 41 | 13 (31.7) | 28 (68.3) |
| Tumour gross appearance | Fungating | 38 | 16 (42.1) | 22 (57.9) | 0.183 |
| Ulcerative | 17 | 6 (35.3) | 11 (64.7) |
| Infiltrative | 5 | 0 (0) | 5 (100) |
| Tumour histologic grade | Low grade | 27 | 11 (40.7) | 16 (59.3) | 0.55 |
| High grade | 33 | 11 (33.3) | 22 (66.7) |
| Association with bilharziasis | Yes | 32 | 9 (28.1) | 23 (71.9) | 0.142 |
| No | 28 | 13 (46.4) | 15 (53.6) |
| Tumour multifocality | Unifocal | 11 | 4 (36.4) | 7 (63.6) | >0.99 |
| Multifocal | 49 | 18 (36.7) | 31 (63.3) |
| T stage | T1-T2 | 22 | 11 (50) | 11 (50) | 0.33 |
| T3-T4 | 38 | 11 (28.9) | 27 (71.1) |
| Nodal status | Positive | 16 | 8 (50) | 8 (50) | 0.2 |
| Negative | 44 | 14 (31.8) | 30 (68.2) |
| Lympho-vascular invasion | Present | 33 | 10 (30.3) | 23 (69.7) | 0.26 |
| Absent | 27 | 12 (44.4) | 15 (55.6) |
| Perineural invasion | Present | 22 | 4 (18.2) | 18 (81.8) | 0.024 |
| Absent | 38 | 18 (47.4) | 20 (52.6) |
p-value less than 0.05 statistically significant
Relation between Ki-67 immunostaining and clinicopathologic features.
| Variable | Total N (60) | Ki-67 immunostaining N (%) | p-value |
|---|
| 0 (5) | 1 (14) | 2 (20) | 3 (21) |
|---|
| Age (Years) | <60 | 20 | 2 (10) | 6 (30) | 5 (25) | 7 (35) | 0.73 |
| ≥60 | 40 | 3 (7.5) | 8 (20) | 15 (37.5) | 14 (35) |
| Sex | Male | 54 | 5 (9.3) | 13 (24.1) | 17 (31.5) | 19 (35.2) | 0.73 |
| Female | 6 | 0 (0) | 1 (16.7) | 3 (50) | 2 (33.3) |
| Tumour size | <4 cm | 19 | 0 (0) | 6 (31.6) | 4 (21.1) | 9 (47.4) | 0.133 |
| ≥4 cm | 41 | 5 (12.2) | 8 (19.5) | 16 (39) | 12 (29.3) |
| Tumour gross appearance | Fungating | 38 | 3 (7.9) | 8 (21) | 14 (36.8) | 13 (34.2) | 0.13 |
| Ulcerative | 17 | 2 (11.8) | 3 (17.6) | 5 (29.4) | 7 (41.2) |
| Infiltrative | 5 | 0 (0) | 3 (60) | 1 (20) | 1 (20) |
| Tumour Histologic grade | Low grade | 27 | 4 (14.8) | 13 (48.1) | 6 (22.2) | 4 (14.8) | <0.001 |
| High grade | 33 | 1 (3) | 1 (3) | 14 (42.4) | 17 (51.5) |
| Association with Bilharziasis | Yes | 32 | 1 (3.1) | 7 (21.9) | 15 (46.9) | 9 (28.1) | 0.072 |
| No | 28 | 4 (14.3) | 7 (25) | 5 (17.9) | 12 (42.9) |
| Tumour multifocality | Unifocal | 11 | 2 (18.2) | 2 (18.2) | 3 (27.3) | 4 (36.4) | 0.6 |
| Multifocal | 49 | 3 (6.1) | 12 (24.5) | 17 (34.7) | 17 (34.7) |
| T stage | T1-T2 | 22 | 2 (9) | 6 (27.3) | 6 (27.3) | 8 (36.4) | 0.68 |
| T3-T4 | 38 | 3 (7.9) | 8 (21) | 14 (36.8) | 13 (34.3) |
| Nodal status | Positive | 16 | 1 (6.2) | 3 (18.8) | 6 (37.5) | 6 (37.5) | 0.93 |
| Negative | 44 | 4 (9.1) | 11 (25) | 14 (31.8) | 15 (34.1) |
| Lympho-vascular invasion | Present | 27 | 0 (0) | 5 (18.5) | 6 (22.2) | 16 (59.3) | 0.002 |
| Absent | 33 | 5 (15.2) | 9 (27.3) | 14 (42.4) | 5 (15.2) |
| Perineural invasion | Present | 22 | 3 (13.6) | 5 (22.7) | 7 (31.8) | 7 (31.8) | 0.73 |
| Absent | 38 | 2 (5.3) | 9 (23.7) | 13 (34.2) | 14 (36.8) |
p-value less than 0.05 statistically significant
Relation between HER2/neu positivity and Ki-67 expression.
| HER2/neu positivity | Ki-67 immunostaining N (%) | Total | p-value |
|---|
| 0 | 1 | 2 | 3 |
|---|
| Positive | 3 (7.9) | 9 (23.7) | 13 (34.2) | 13 (34.2) | 38 | 0.99 |
| Negative | 2 (9.1) | 5 (22.7) | 7 (31.8) | 8 (36.4) | 22 |
p-value less than 0.05 statistically significant
Discussion
In Egypt, bladder cancer is considered one of the public health problems, being the most common (17% of all cancer cases) in males; while in females it accounts for 5%, with a male/female ratio of 3.5:1 [17]. Urothelial carcinoma is the most common type of bladder cancer accounting for about 80% to 90% of bladder cancer worldwide. Other types of bladder cancer such as squamous cell carcinoma and adenocarcinoma are much less common [18]. A worldwide considerable attention has been given to the identification of prognostic biomarkers in urothelial carcinoma [19]. HER2/neu is considered one of the most frequently amplified oncogenes in bladder cancer [20]. It appears to play role in the tumour pathogenesis; however, its expression is variable between different studies [21]. The prognostic value of HER2/neu in bladder carcinoma has not yet been established; however, the success of trastuzumab therapy in patients with breast carcinoma has stimulated interest in exploring the potentiality of using this therapy for patients with bladder carcinoma [22].
There are wide variations in HER2/neu expression in urinary bladder carcinoma in the reports of different investigators, so this study showed examined the expression of HER2/neu in Egyptian cases with urothelial carcinoma [22-24]. In this study, HER2/neu expression was seen in 38 cases (representing 63.3%). In an independent study using 111 bladder carcinoma samples, demonstrated that HER2/neu over-expression was observed in 22% of the analysed cohort [23]. This percentage is widely variable and could reach in some cases a record ratio of 74% [24].
Unlike a large section of the studies in the literature that reported significant relation between HER2/neu expression and most of the important prognostic factors, in this study the relation between HER2/neu expression and all the studied clinicopathologic features was insignificant except for the presence of perineural invasion (p=0.024) [25]. The usage of different antibodies applied for immunohistochemistry, in addition to the inconsistent criteria set for detecting IHC positivity may be the cause that the literature in this field is extremely difficult to compare, and significant conclusions are hard to comment upon, also considering geographical, racial, and genetic differences are very important factors during results comparison. Ki-67 is a biologic marker that can be measured objectively in cancer and its expression can be compared after therapeutic intervention [26]. In addition, Ki-67 expression can help in discrimination between cases of bladder dysplasia and carcinoma and helps in identifying cases of high-grade carcinoma [27].
Immunohistochemical assays of proliferative markers, such as the Ki-67 is currently used worldwide by over 90% of pathologists to diagnose bladder cancer [28]. However, the role of Ki-67 in the prognosis of bladder cancer remains controversial. Previous studies showed significant relations between Ki-67 expression and the histologic grade of the tumour as well as the presence of lympho-vascular invasion which is similar to the results in this study. It also reported nonsignificant relation between Ki-67 expression and HER2/neu positivity as reported in this study [25,29].
Limitation(s)
The results of this study are preliminary and were dependent upon relatively small sample size.
Conclusion(s)
HER2/neu immunohistochemical expression did not correlate with most of the studied clinicopathologic items and had no significant correlation with the proliferative marker (Ki67), so it seems to be not as effective prognostic marker as supported by other previous studies in urothelial carcinoma and it may be of no therapeutic role as claimed by others.
p-value less than 0.05 statistically significant
p-value less than 0.05 statistically significant
p-value less than 0.05 statistically significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 16, 2020
Manual Googling: Sep 03, 2020
iThenticate Software: Jan 16, 2021 (22%)
[1]. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, GLOBOCAN 2012: Estimated cancer Incidence, mortality and prevalence worldwide in 2012 v1.0. IARC Cancer Base No. 11. Lyon, France. International Agency for Research on Cancer. World Health Organization [Google Scholar]
[2]. Lara J, Brunson A, Keegan TH, Malogolowkin M, Pan CX, Yap S, Determinants of survival in adolescents and young adults with urothelial bladder cancer: Results from the California Cancer RegistryJ Urol 2016 196(5):1378-82.10.1016/j.juro.2016.05.08227208515 [Google Scholar] [CrossRef] [PubMed]
[3]. El Moneim HMA, Tawfik HM, El Sherbiny YM, Tawfiek ER, Analysis of Her2/neu overexpression and amplification in urothelial carcinoma of the bladder associated with Cox-2 overexpressionInt J Cancer Res 2011 7(1):08-24.10.3923/ijcr.2011.8.24 [Google Scholar] [CrossRef]
[4]. Kumar V, Abbas AK, Fausto N, Robbins and Cotran, pathologic basis of disease, Her2/ Neu and Ki-67Immunohistochemical Expression in Transitional Cell Carcinoma of The Urinary Bladder 2010 8th edPhiladelphia USAElsevier:976-80. [Google Scholar]
[5]. Heyns CF, Merwe AV, Bladder cancer in AfricaCan J Urol 2008 15(1):3899-908. [Google Scholar]
[6]. Lagwinski N, Thomas A, Stephenson A, Campbell S, Hoschar AP, El-Gabry E, Squamous cell carcinoma of the bladder clinicopathologic analysis of 45 casesAm J Surg Pathol 2007 31(12):1777-6.87.10.1097/PAS.0b013e31805c9cd918043032 [Google Scholar] [CrossRef] [PubMed]
[7]. Li R, Heydon K, Hammond ME, Grignon DJ, Roach M 3rd, Wolkov HB, Ki-67staining index predicts distant metastasis and survival in locally advanced prostate cancer treated with radiotherapy: An analysis of patients in radiation therapy oncology group protocolClin Cancer Res 2004 10(12Pt1):4118-24.10.1158/1078-0432.CCR-1052-0315217948 [Google Scholar] [CrossRef] [PubMed]
[8]. Gönül II, Akyürek N, Dursun A, Küpeli B, Relationship of Ki67, TP53, MDM-2 and BCL-2 expressions with WHO 1973 and WHO/ISUP grades, tumour category and overall patient survival in urothelial tumours of the bladderPathol Res Pract 2008 204:707-17.10.1016/j.prp.2008.03.01118572327 [Google Scholar] [CrossRef] [PubMed]
[9]. Olayioye MA, Update on HER-2 as a target for cancer therapy: Intracellular signaling pathways of ErbB2/HER-2 and family membersBreast Cancer Res 2001 3(6):385-89.10.1186/bcr32711737890 [Google Scholar] [CrossRef] [PubMed]
[10]. Nicholson RI, Gee JM, Harper ME, EGFR and cancer prognosisEur J Cancer 2001 37(4):S9-15.10.1016/S0959-8049(01)00231-3 [Google Scholar] [CrossRef]
[11]. Tinoco G, Warsch S, Glück S, Avancha K, Montero AJ, Treating breast cancer in the 21st century: Emerging biological therapiesJ Cancer 2013 4(2):117-32.10.7150/jca.492523386910 [Google Scholar] [CrossRef] [PubMed]
[12]. Sasaki Y, Sasaki T, Kawai T, Morikawa T, Matsusaka K, Kunita A, HER2 protein overexpression and gene amplification in upper urinary tract urothelial carcinoma-an analysis of 171 patientsInternational Journal of Clinical and Experimental Pathology 2014 7(2):699-708. [Google Scholar]
[13]. Ikeda S, Hansel DE, Kurzrock R, Beyond conventional chemotherapy: Emerging molecular targeted and immunotherapy strategies in urothelial carcinomaCancer Treatment Reviews 2015 41(8):699-706.10.1016/j.ctrv.2015.06.00426138514 [Google Scholar] [CrossRef] [PubMed]
[14]. Kumar M, Immunohistochemical expession of markars of ki67 and her2 neu and its corelation with clinicopathological parameters of urothelial tumoursInternational Journal of Current Research 2016 8(12):43704-08. [Google Scholar]
[15]. Abd El-Aty S, Amal A, Gehan M, Hisham Y, Adel F, Her-2/Neu Overexpression in invasive bladder. Carcinoma among a cohort of Egyptian patientsWorld J Nephrol Urol 2013 2(2):70-75. [Google Scholar]
[16]. Lujia W, Chenchen F, Guanxiong D, Zhongwen Z, Haowen J, Zhong W, Relationship of TP53 and Ki67 expression in bladder cancer under WHO 2004 classificationJbuon 2013 18(2):420-24. [Google Scholar]
[17]. El-Sharkawi F, El Sabah M, Hassan Z, Khaled H, The biochemical value of urinary metalloproteinases 3 and 9 in diagnosis and prognosis of bladder cancer in EgyptJ Biomed Sci 2014 21(1):7210.1186/s12929-014-0072-425135219 [Google Scholar] [CrossRef] [PubMed]
[18]. Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF, The global epidemiology of bladder cancer: A join point regression analysis of its incidence and mortality trends and projectionSci Rep 2018 8:112910.1038/s41598-018-19199-z29348548 [Google Scholar] [CrossRef] [PubMed]
[19]. Zhao J, Xu W, Zhang Z, Song R, Zeng S, Sun Y, Prognostic role of HER2 expression in bladder cancer: A systematic review and meta-analysisInt Urol Nephrol 2015 47:87-94.10.1007/s11255-014-0866-z25384433 [Google Scholar] [CrossRef] [PubMed]
[20]. Simon R, Atefy R, Wagner U, Forster T, Fijan A, Bruderer J, HER-2 and TOP2A co-amplification in urinary bladder cancerInt J Cancer 2003 107:764-72.10.1002/ijc.1147714566826 [Google Scholar] [CrossRef] [PubMed]
[21]. Wester K, Sjostrom A, de la Torre M, Carlsson J, Malmstrom PU, HER-2 a possible target for therapy of metastatic urinary bladder carcinomaActa Oncol 2002 41(3):282-88.10.1080/0284186026008883612195748 [Google Scholar] [CrossRef] [PubMed]
[22]. El Gehani K, Al-Kikhia L, Emaetig F, Syrjänen K, Al-Fituri O, Elzagheid A, Over- expression of HER-2 is associated with the stage in carcinomas of the urinary bladderLibyan J Med 2012 710.3402/ljm.v7i0.1469422408683 [Google Scholar] [CrossRef] [PubMed]
[23]. Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, HER2 as a target in invasive urothelial carcinomaCancer Med 2015 4(6):844-52.10.1002/cam4.43225720673 [Google Scholar] [CrossRef] [PubMed]
[24]. Zhau HE, Zhang X, Von Eschenbach AC, Scorsone K, Babain RJ, Ro JY, Amplification and expression of the c-erb B-2/neu proto-oncogene in human bladder cancerMol Carcinog 1990 3(5):254-57.10.1002/mc.29400305031978777 [Google Scholar] [CrossRef] [PubMed]
[25]. Kumar GS, Gokhan E, De Munter S, Bollen M, Vagnarelli P, Peti W, The Ki-67 and RepoMan mitotic phosphatases assemble via an identical, yet novel mechanismeLife 2016 5:e1653910.7554/eLife.1653927572260 [Google Scholar] [CrossRef] [PubMed]
[26]. Sarkis AS, Dalbagni G, Cordon-Cardo C, Zhang ZF, Sheinfeld J, Fair WR, Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: A marker for disease progressionJ Natl Cancer Inst 1993 85:53-59.10.1093/jnci/85.1.537677935 [Google Scholar] [CrossRef] [PubMed]
[27]. Badawi MA, El-Sharkawy SL, Abbas NF, Abdel-Aal WE, Image analysis and Ki-67 expression in urothelial dysplasia and carcinomaJ Arab Soc Med Res 2018 13:144-50.10.4103/jasmr.jasmr_32_18 [Google Scholar] [CrossRef]
[28]. Lopez-Beltran A, Algaba F, Berney DM, Boccon-Gibod L, Camparo P, Griffiths D, Handling and reporting of transurethral resection specimens of the bladder in Europe: A web-based survey by the European Network of Uropathology (ENUP)Histopathology 2011 58:579-85.10.1111/j.1365-2559.2011.03784.x21348893 [Google Scholar] [CrossRef] [PubMed]
[29]. Thakur B, Kishore S, Dutta K, Kaushik S, Bhardwaj A, Role of p53 and Ki-67 immunomarkers in carcinoma of urinary bladderIndian J Pathol Microbiol 2017 60:505-09.10.4103/IJPM.IJPM_246_1729323062 [Google Scholar] [CrossRef] [PubMed]