PTB, as well as smoking, exposure to wood stove smoke, biomass, childhood respiratory tract infections and asthma, have been implicated as risk factors for the development of OVD [3], with smoking being the main one [4]. Approximately, 20% of patients who meet the criteria for airflow obstruction are not smokers, and PTB is considered a possible cause of obstruction [5].
In the lungs, PTB can evolve with destruction of the bronchi and the parenchyma, resulting in scarring, bronchiectasis and fibrosis, which lead to the impairment of lung function, even in adequately treated patients [6]. Functional changes resulting from PTB observed after treatment appear as Restrictive Ventilatory Disorder (RVD), OVD, or Mixed (obstructive-restrictive) Ventilator Disorder (MVD) [3,7,8]. Some studies described that most frequent alteration is OVD [9-12]. However, Santra A et al., showed MVD as the most prevalent (72%) [8]. In Brazil, Maria L et al., observed MVD in 34% and OVD was observed in 34% to 49% by other authors [13-15]. In a multicentre study, without a history of lung disease or smoking prior treatment of PTB, the RVD was the most prevalent (24.7%) [7].
Short-acting BD response, measured by FEV1, in post treatment TB patients, ranged from 7.7 to 21% [16-18]. However, in these studies, individuals with lung disease prior to TB treatment, smokers and former smokers were also included.
The primary aim of this study was to evaluate the response to bronchodilator in OVD in patients after treatment of PTB without history of smoking or previous lung disease.
Materials and Methods
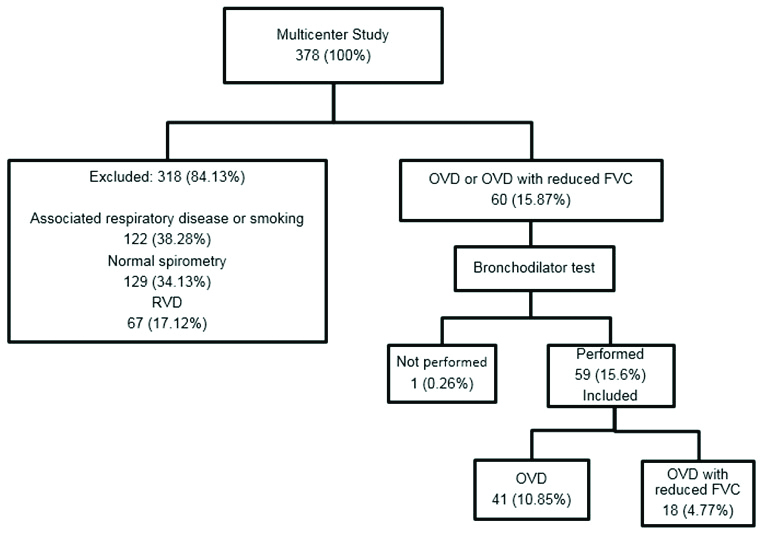
This was a retrospective study that included data of 60 patients. The data was a part of a larger multicentric study (n=378) that was conducted to compare results from spirometry in patients treated for PTB, with or without previous pulmonary diseases. The detailed methodology of the multicentre study had been previously published [7].
The participants were recruited from February 2014-2015 from the Hospital das Clínicas of the Federal University of Minas Gerais (secondary reference for the treatment of TB); the Brazilian Institute for Tuberculosis Research (IBIT), Salvador, Bahia (primary reference); the University Hospital of the Federal University of Grande Dourados, Mato Grosso do Sul (primary reference); and the Clementino Fraga Filho University Hospital of the Federal University of Rio de Janeiro (secondary reference). The study was approved by the Research Ethics Committee of the Federal University of Minas Gerais: CAAE number 14606113.7.0000.5149.
Inclusion criteria: From the 378 patients in the multicentre study, 60 spirometry results from patients with OVD or OVD with reduced FVC, without previous pulmonary diseases (asthma, COPD, interstitial lung disease, bronchiectasis or silicosis) and without smoking history were included in this study.
Exclusion criteria: Patients with normal tests, with previous pulmonary disease and smoking history and RVD were excluded [Table/Fig-1].
Flowchart showing the patient distribution.
RVD: Restrictive ventilatory disorder; OVD: Obstructive ventilatory disorder; FVC: Forced vital capacity
Available data were collected from a standardised questionnaire (socio-demographic and clinical data, gender, age, smoking, alcohol consumption and co-morbidities). The time between the onset of symptoms and the diagnosis of PTB was also assessed. A smoker was considered to be an individual who smoked at least 100 cigarettes or equivalent in his or her lifetime. A former smoker was one whose habit had been discontinued for more than 12 months [19]. In the classification of alcoholism, the CAGE questionnaire was used (CAGE is an acronym referring to four questions: Cut down, Annoyed by criticism, Guilty and Eye-opener) [20].
The diagnosis of pulmonary diseases prior to the treatment of PTB was reviewed by the pulmonologists involved in this study, following the definitions proposed by the Global Initiative for Asthma (GINA) guidelines for Asthma, Global Initiative for Obstructive Chronic Lung Disease (GOLD) for COPD and Pneumological Practice [4,21,22]. Participants were asked about the occurrence of wheezing in childhood, as well as symptoms of rhinitis and atopic dermatitis.
The presence of cough, sputum, wheezing and dyspnoea (classified according to the scale of the modified Medical Research Council-mMRC) [23] was evaluated on the day of spirometry.
In spirometry, the Koko brand spirometer (Pulmonary Data Service, Inc. Company, Louisville, CO, USA) was used. Acceptance and reproducibility criteria for pulmonary function tests followed the recommendations of the American Thoracic Society/European Respiratory Society [24]. The values found were described as absolute values and as a percentage of the predicted values for the Brazilian population [25]. The technicians who performed the tests were certified by the Brazilian Society of Pulmonology. The tests were performed six months after tuberculosis treatment. In the interpretation of spirometry, the following standards were defined: normal spirometry, OVD, OVD with reduced vital capacity (OVD with reduced VC) and RVD [26]. OVD was considered when the FEV1 and FEV1/FVC ratio below the Lower Limit of Normal (LLN) and the FVC within the LLN. FEV1/FVC ratio and FVC below LLN was considered OVD with reduced FVC. RVD was inferred when FEV1/FVC ratio equal or above LLN and FVC below LLN and clinical and chest radiographs data suggested restriction [26]. To classify the severity of the obstruction in the spirometry, we used a percentage of predicted of FEV1 and FEV1/FVC, with ≥60% mild, 41-59% moderate, and ≤40% severe; for restriction we used percent prediction of FVC ≥60% mild, 51-59% moderate, and ≤50% severe [26]. Salbutamol, 400 μcg, was used as a bronchodilator during the tests. The bronchodilator response was considered positive when observed an increase in FEV1 and/or FVC ≥12% (as a percentage of change from baseline) and ≥200 mL (in absolute values) after administering salbutamol in four separate doses of 100 mcg [27].
Radiological Evaluation
Chest radiographs were performed on dates close to the spirometry, evaluated by radiologists and classified by pulmonologists. Unchanged chest radiographs were classified as normal. For the others, the National Tuberculosis Association (NTA) [28] classification was used: NTA I or minimum; NTA II or moderately advanced. The lesion may be in one or both lungs, its extent should not exceed the volume corresponding to an entire lung if the lesions are not confluent, and, in the presence of confluent lesions, they should not occupy more than the equivalent of one third of the lung; NTA III or very advanced that exceeds the moderate limit.
Statistical Analysis
The variables were entered in a spreadsheet developed in the Excel program and analysed using the Statistical Package for the Social Sciences program, version 22.0 (SPSS Inc., Chicago, IL, USA). The distribution of the variables was assessed using the Kolmogorov-Smirnov test. Continuous variables were expressed as mean and standard deviation or median and interquartile range, while categorical variables were expressed as absolute and relative frequency. To verify the association between socio-demographic, clinical and radiological variables and the response to BD, the Fisher-Exact Test was used. The level of significance was set at p<0.05.
Results
A total of 59 patient’s data with OVD, and OVD with reduced FVC, were included in the final analyses [Table/Fig-1]. The socio-demographic, clinical and radiological characteristics of the participants are shown in [Table/Fig-2]. There was a higher frequency of males, incomplete primary education and married individuals. The mean age was 47 years. Systemic Arterial Hypertension (SAH) was the most prevalent co-morbidity. Alcoholism was absent in a majority of patients (76.3%). In clinical evaluation, dyspnoea mMRC 0-1 (91.5%) was the most frequent symptom. In the radiological evaluation, a majority of participants (66.1%) presented little or no alteration after treatment. The mean time between onset of symptoms and diagnosis of TB was three months.
Clinical, socio-demographic and radiological characteristics.
| Characteristic | Features | n | % |
|---|
| Gender | Male | 32 | 54.2 |
| Female | 27 | 45.8 |
| Age range (in years) | 18-29 | 12 | 20.3 |
| 30-49 | 22 | 37.3 |
| 50-59 | 11 | 18.6 |
| 60 and above | 14 | 23.8 |
| Race/colour | White | 7 | 11.9 |
| Non-white | 52 | 88.1 |
| Schooling (a) | Complete or incomplete elementary education | 29 | 49.2 |
| High school or higher education | 16 | 27.1 |
| Marital status (b) | Married/Stable union | 31 | 52,5 |
| Others | 27 | 45.8 |
| Alcoholismc | Yes | 13 | 22.0 |
| No | 45 | 76.3 |
| Co-morbidities | HIV | 2 | 3.4 |
| Hypertension | 12 | 20.3 |
| Cardiopathy | 1 | 1.7 |
| Diabetes mellitus | 2 | 3.4 |
| Kidney disease | 4 | 6.8 |
| Respiratory symptoms | Degree of dyspnea, mMRC 0-1 | 54 | 91.5 |
| Cough | 16 | 27.1 |
| Sputum | 9 | 15.3 |
| Wheezing | 3 | 5.1 |
| NTA classification (d) | Normal or NTA-I | 39 | 66.1 |
| NTA II or III | 15 | 25.4 |
| Time of symptoms until diagnosis (e) | <30 days | 12 | 20.3 |
| 30 days or more | 37 | 62.7 |
*mean±standard deviation, mMRC: modified medical research council; NTA: National tuberculosis association; a: n=14, no available data; b: n=1, no available data; c: n=1, no available data; d: n=5, no available data; e: n=10, no available data
The absolute and percentage predicted values obtained in spirometry before and after BD are presented in [Table/Fig-3]. In spirometry, mild OVD was observed in 61.02% of the patients, followed by moderate OVD with reduced FVC in 20.3% [Table/Fig-4].
Spirometric variables (n=59).
| Parameter | Mean (SD) | Mean (SD) % of predicted |
|---|
| FVC (L) | 3.3 (1.1) | 83.8 (17.6) |
| FVC-BD (L) | 3.4 (1.1) | 84.7 (18.7) |
| FEV1 (L) | 2.3 (0.8) | 70.9 (19.9) |
| FEV1-BD (L) | 2.5 (0.9) | 74.8 (19.7) |
| FEV1/FVC % | | 69.2 (7.5) |
SD: Standard deviation; L: Litre; FVC: forced vital capacity; FVC-BD: Forced vital capacity after use of the bronchodilator; FEV1: forced expiratory volume in the first second; FEV1-BD: Forced expiratory volume after use of the bronchodilator; FEV1/FVC%: ratio forced expiratory volume in the first second to forced vital capacity in % of predicted
Classification of the seriousness of ventilatory disorder.
| Disorder | n | % | Grade | n | % |
|---|
| OVD | 41 | 69.5 | Mild | 36 | 61.02 |
| Moderate | 4 | 6.8 |
| Severe | 1 | 1.7 |
| OVD with reduced CVF | 18 | 30.5 | Mild | 4 | 6.8 |
| Moderate | 12 | 20.3 |
| Severe | 2 | 3.38 |
| Total | 59 | 100 | | 59 | 100 |
OVD: Obstructive ventilatory disorder; FVC: Forced vital capacity
In the results of spirometry after BD use, 13/59 (22.03%) of the patients presented a positive response, which was evidenced in FEV1 in 7/59 (11.9%), and FVC in 5/59 (8.5%). Only 1/59 (1.7%) of the patients presented a confirmed response in FEV1 and FVC simultaneously [Table/Fig-5].
Bronchodilator response in the spirometry (n=59).
| Bronchodilator response | n | % |
|---|
| FVC | 5 | 8.47 |
| FEV1 | 7 | 11.87 |
| FVC and FEV1 | 1 | 1.69 |
| Total | 13 | 22.03 |
FVC: Forced vital capacity; FEV1: Forced expiratory volume in the first second
After Fisher-Exact Test analysis, no significant associations between socio-demographic, clinical and radiological variables, and BD response were observed [Table/Fig-6].
Associated factors for the bronchodilator response.
| Variables of study subjects | Features | Response to BD | p-value |
|---|
| No | Yes |
|---|
| n=46 | n=13 |
|---|
| n | % | n | % |
|---|
| Gender | Male | 25 | 54.3 | 7 | 53.8 | 0.97 |
| Female | 21 | 45.7 | 6 | 46.2 |
| Age range | Up to 29 years | 10 | 21.7 | 2 | 15.4 | 0.56 |
| 30-49 years | 16 | 34.8 | 6 | 46.2 |
| 50-59 years | 10 | 21.7 | 1 | 7.7 |
| 60 years and above | 10 | 21.7 | 4 | 30.8 |
| BMI, Kg/m2 | <25 | 37 | 80.4 | 9 | 69.2 | 0.39 |
| ≥25 | 9 | 19.6 | 4 | 30.8 |
| Race/color | White | 5 | 10.9 | 2 | 15.4 | 0.65 |
| Non-white | 41 | 89.1 | 11 | 84.6 |
| Schooling | Complete or incomplete elementary education | 23 | 63.9 | 6 | 66.7 | 0.88 |
| High school or higher education | 13 | 36.1 | 3 | 33.3 |
| Marital status | Married/Stable union | 26 | 57.8 | 5 | 38.5 | 0.22 |
| Others | 19 | 42.2 | 8 | 61.5 |
| Alcoholism | Yes | 10 | 21.7 | 3 | 25.0 | 0.81 |
| No | 36 | 78.3 | 9 | 75.0 |
| Respiratory symptoms | Degree of dyspnea: mMRC 0-1 | 44 | 95.7 | 10 | 90.9 | 0.48 |
| Cough | 12 | 26.1 | 4 | 33.3 |
| Sputum | 5 | 10.9 | 4 | 33.3 |
| Wheezing | 3 | 6.5 | 0 | 0.0 |
| NTA classification* | Normal or NTA-I | 31 | 73.8 | 8 | 66.7 | 0.63 |
| NTA II or III | 11 | 26.2 | 4 | 33.3 |
| Time of symptoms until diagnosis | <30 days | 9 | 23.1 | 3 | 30.0 | 0.65 |
| >30 days | 30 | 76.9 | 7 | 70.0 |
BMI: Body mass index; llll; mMRC: modified medical research council; NTA: National tuberculosis association; *n=5 NTA no available data: 4 without BD and 1 BD response; Fischer exact test used to calculate the significance; p-value<0.05 to be considered the significance level
Discussion
The main findings of this study, conducted in three regions of Brazil, showed that 13/59 (22.03%) of the participants with airflow obstruction after PTB treatment presented a positive response to bronchodilator in spirometry. It should be noted that patients with a history of lung disease or smoking prior to treatment of PTB were excluded.
The pathophysiology leading to the development of airflow obstruction in PTB is multifactorial in nature [3,6]. Endobronchial involvement may result in localised and diffuse bronchial obstruction, fibrosis, and increased airway resistance. Parenchymal lung destruction may also affect lung compliance, resulting in an increased tendency for peripheral airway collapse and subsequent air entrapment [6]. Thus, the outcome will be a compromised lung function, evolving into airflow obstruction [3,29]. The BD response in patients with treated PTB may be justified by their performance in the mechanisms involved in obstruction, such as mucosal oedema, hypertrophy and hyperplasia of the mucous glands, increased secretion of mucus and hypertrophy of the smooth muscle which alters the airway caliber, increases its resistance and reduces airflow [3,6].
In addition to factors associated with infection, individual characteristics (such as those related to genetics, systemic inflammatory response, and initial extent of PTB lesions) are involved in airflow obstruction [30]. A recent study indicated association of the Matrix-Metalloproteinase (MMP) system in the remodeling mechanisms of pulmonary Extracellular Matrix (ECM), which contributes to the development of airflow obstruction in TB [6]. MMPs play important roles in normal lung immunity. However, in diseased, inflamed or remodeling and repair tissues, these are expressed in excess, which may contribute to the emergence of destructive pulmonary diseases, and proteolytic activity leading to the degradation of the lung ECM [31]. This heterogeneity of lesions affecting the lung should be better studied, especially in relation to pathophysiology and inflammatory phenomena.
In the index study, there was a greater predominance of OVD in relation to OVD with reduced FVC, 69.5% versus 30.5%, respectively, with OVD being the most frequent. Sailaja K and Rao HN also found similar results in their series, with OVD being 62.5% and mixed pattern abnormalities 21.42%; however, the pattern of moderate obstructive abnormalities was the most frequent [32].
In the present study, a positive response to BD was observed in 22.03% of the patients, which is close to the variation described in the literature, from 14 to 21% [16-18]. However, different from what had been observed in previous studies, smokers, ex-smokers and those with other pulmonary diseases prior to the treatment of PTB were excluded, thus reducing confounding factors in the results and reinforcing that the response to BD was probably related to airflow obstruction by the PTB. The therapeutic effect of BD in Chronic Obstructive Pulmonary Disorder (COPD) manifests itself clinically, with a significant improvement in dyspnea due to decreased resistance of small airways and pulmonary hyper-inflation, increasing exercise capacity and improving quality of life [4].
Limitation(s)
The limitation present in this study is the restricted number of participants that were included.
Conclusion(s)
Response to BD was observed in a quarter of patients with OVD who were treated by PTB and without previous pulmonary diseases or smoking history, is likely due to a structural change. The specific treatment of these patients should be studied in future studies.
Future studies should investigate the medium and long-term benefits of BD in the clinical and functional improvement of these patients. Studies should be performed in order to verify if this same mechanism occurs in airflow obstruction post PTB.
*mean±standard deviation, mMRC: modified medical research council; NTA: National tuberculosis association; a: n=14, no available data; b: n=1, no available data; c: n=1, no available data; d: n=5, no available data; e: n=10, no available data
SD: Standard deviation; L: Litre; FVC: forced vital capacity; FVC-BD: Forced vital capacity after use of the bronchodilator; FEV1: forced expiratory volume in the first second; FEV1-BD: Forced expiratory volume after use of the bronchodilator; FEV1/FVC%: ratio forced expiratory volume in the first second to forced vital capacity in % of predicted
OVD: Obstructive ventilatory disorder; FVC: Forced vital capacity
FVC: Forced vital capacity; FEV1: Forced expiratory volume in the first second
BMI: Body mass index; llll; mMRC: modified medical research council; NTA: National tuberculosis association; *n=5 NTA no available data: 4 without BD and 1 BD response; Fischer exact test used to calculate the significance; p-value<0.05 to be considered the significance level