Introduction
Uterine Leiomyoma is a highly morbid condition with an increasing incidence in the Asian Indian ethnicity. The pathogenesis is multifactorial and not clearly delineated, making non-surgical treatment of limited success, thus making surgical intervention prevalent. Derangement of endocrinological parameters is the most evident aspect and cause of this condition. But the root genetic cause of this hormonal imbalance has seldom been explored in Indian women suffering from uterine leiomyoma.
Aim
To explore the association of Single Nucleotide Polymorphism (SNPs) rs3020449, rs4986938 and rs743572 of ER alpha, ER beta and CYP17 genes respectively in women having uterine fibroid visiting a Tertiary Care Hospital in North India.
Materials and Methods
Hundred patients diagnosed with uterine leiomyoma were selected from Gynaecology Out Patient Department (OPD) of a Tertiary Care Hospital in North India and equal age matched healthy women were taken as controls randomly, with a condition that they have no close blood relative with uterine leiomyoma. The blood was collected for DNA extraction and RFLP based polymorphism detection. Bands were visualised in agarose gel for Estrogen Receptor alpha (ER alpha), ER beta and CYP17 genes. Statistical analysis was performed using Graph Pad Prism 6.0. Hardy Weinberg equilibrium was tested using Chi-square goodness of fit test. Nominal variables were analysed using Fisher-exact test. Data is presented as Mean±SEM and p-value of <0.05 was considered significant.
Results
Early age at menarche (cases vs control 11.51±0.19 year vs 12.04±0.12, p=0.03) and less number of previous pregnancies (cases vs control 1.06±0.09 vs 1.41±0.12, p=0.01), which are known risk factors for the uterine fibroids, was reconfirmed in this study. The genotype distribution of all subjects studied in above genes followed Hardy Weinberg equilibrium and there was no significant difference in genotype frequencies between cases and controls [ER alpha rs9322331 C allele (cases vs controls; 62% vs 64%), T allele (38% vs 36%); ER beta rs4986938 G allele (64% vs 59%), A allele (36% vs 41%); CYP17 rs743572 T allele (44.95% vs 46.53%), C allele (55.05% vs 53.47%) p-value not significant in any]. However, there was an increased propensity of TC genotype of CYP17 rs743572 towards obesity (p<0.05).
Conclusion
The allelic frequencies of all the three SNPs were similar in cases and controls indicating that they do not affect susceptibility to disease. However, the association of TC allele of CYP17 SNP with higher BMI needs further analysis.
Cytochrome P450, Oestrogen receptor genes, Uterine fibroids
Introduction
Uterine leiomyomas have a prevalence of 20-25% in women of reproductive age causing dysmenorrhoea, menorrhagia, reduced fertility, obstructive symptoms and rarely malignant presentations [1]. The unclear pathogenesis makes nonsurgical treatment unsatisfactory [2]. Among the various risk factors [2], increase in oestrogen and progesterone levels is foremost. These hormones are involved in tumour growth but with mechanisms different from endometrial growth during menstrual cycles, menarche or pregnancy [3]. Each tumour of uterine leiomyoma has its own intrinsic growth rate making the pathogenesis yet more confusing.
Growth of leiomyoma is mostly related to oestrogen and its receptors (ER alpha and beta) [4]. A 17 beta estradiol is believed to be the predominant endogenous activator of ER mediated cell processes [5].
The increase in oestrogen levels in uterine leiomyoma could be attributed to high body mass index/obesity. Oestrogens increases the mitotic rate of uterine cells augment the various growth factors mediated signalling pathways [6]. Several studies have found that mRNA and protein expression levels as well as the content of ER alpha and ER beta receptors are higher in leiomyoma as compared to those in normal myometrium [7-10]. But, as oestrogen influences our appetite, metabolism and body fat distribution, whether obese women have increased oestrogen or increased oestrogen level leads to obesity becomes the perpetual chicken-egg controversy.
Ishikawa H et al., suggested that oestrogens can maintain Progesterone Receptor (PR) levels and thus, progesterone through its receptor may promote leiomyoma growth [11]. In the adrenal cortex, cholesterol is converted to pregnelonone which is the mediator of progesterone. The cytochrome P450 C17 alpha enzyme present in zona reticularis of adrenal cortex is a key enzyme in steroidogenesis pathway responsible for 17 alpha hydroxylase and 17, 20 lyase activities required for conversion of cholesterol to oestrogen.
CYP17A1 located on chromosome 10q24.3 hosts a Single Nucleotide Polymorphism (SNP) on its 5’UTR region (T to C at position 1931) converting allele 1 to allele 2. A2 allele has been seen to increase serum oestrogen and progesterone levels. Studies on this SNP on CYP17A1 gene could lead to understanding how oestrogen levels could be higher in certain women than others.
Oestrogen receptor alpha gene, located on chromosome 6 has a T/C SNP rs 2234693 on intron 1 and exon 2 boundary of oestrogen receptor alpha gene which has been associated with breast cancer, osteoporosis, endometriosis and fibroids in various ethnic groups [12-14]. However, there was no association of ER alpha SNPs rs9322331, rs17847075 in some ethnic groups based in Iran [15] and Brazil [16].
Oestrogen receptor beta gene, located on chromosome 14, has some genetic variants which could modify susceptibility to endometriosis and influence fertility [12]. Another study found that there is raised expression level of ER beta in leiomyomas but SNPs in the promoter region of human ER beta gene are not associated with its development [17].
So, this study was planned to know the relation of SNP rs4986938 of ER beta, SNP rs9322331 of ER alpha and SNP rs743572 of CYP17A gene with uterine leiomyoma in women attending a Tertiary Care Hospital in North India. This could shed light on disease pathophysiology and help in diagnosis and prognosis and non-surgical attempt to treatment.
Materials and Methods
This case control study was conducted in a Tertiary Care Hospital in North India, between June 2014 to July 2015, on 100 Out Patient Department (OPD) patients ultrasonographically diagnosed with uterine leiomyoma in the Gynaecology Department of the hospital. Controls were healthy age-matched women selected randomly, with a condition that they had no first degree relative with uterine leiomyoma. Cases with doubtful ultrasonography regarding diagnosis of uterine fibroid were excluded from the study.
Institutional ethical clearance was obtained vide letter no. IEC/VMMC/SJH/Thesis/Nov-13/106 dated 24.12.2013 and informed written consent was taken from each subject.
Sample Size Calculation
Sample size was calculated using the formula:
nA=(PA (1-PA)/K +PB (1-PB)) (Zα/2 +Z1-β/(PA-PB))2K is nA/nB ratio (0.25), nA=number of cases, nB=number of controls, α (0.05), CI (95%), β (0.02), power (80%), σ (standard deviation). The total sample size calculated with 5% margin of error and 80% confidence level is 198 (99 each cases and controls).
Venous blood was collected in EDTA vial for genotyping. Genomic DNA was extracted manually by the chloroform-phenol method from the blood collected in EDTA vial. The extracted DNA was dissolved and stored in TE buffer. The genes were amplified by Polymerase Chain Reaction (PCR) in MJ Research PTC-100 (peltier thermal cycle). PCR products were analysed in 2% agarose gel in 1X TAE buffer system.
Primers were designed and checked for compatibility using the IDT software [18]. Restriction enzyme was selected and checked using NEB enzyme cutter version 2.0 [19]. These are as follows:
1) CYP17A rs743572
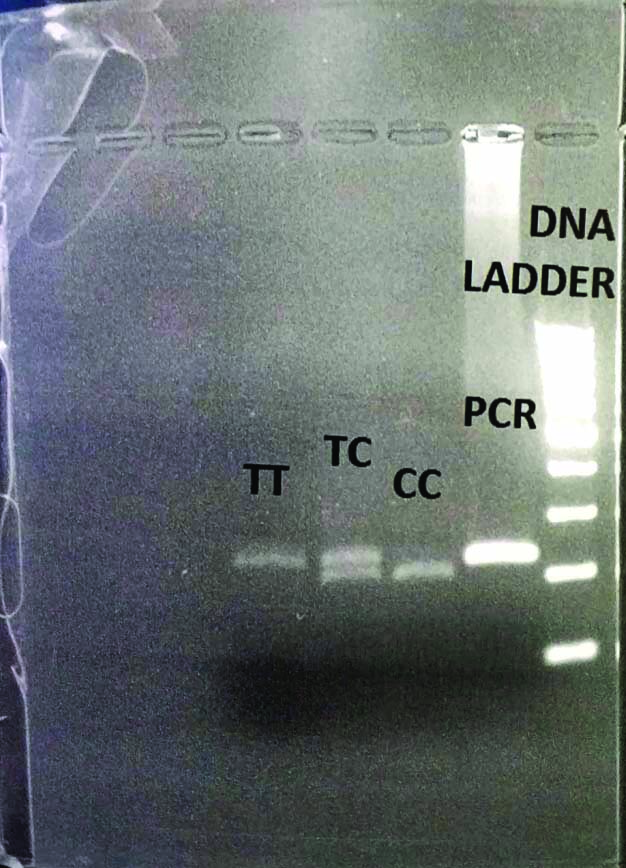
5’-CAT TCG CAC TCT GGA GTC-3’ as forward and 5’-AGG CTC TTG GGG TAC TTG-3’ as reverse, MspA11, TT-414bp, CC-290bp, 124bp.
2) ER alpha rs9322331
5’-CAT CTA CTC CTA TGT CTG GT-3’ as forward and 5’-CGT GTA GAC TGA AGG GCA T-3’ as reverse, HaeIII, TT-227bp, CC-205bp, 22bp.
3) ER beta rs4986938
5’-ACA GAG GCT GAC AAG ACA TCG-3’ as forward and 5’-GGC CAT TGA GTG TGG AAA CG-3’ as reverse primer which amplified a 631 bp, AluI, GG-577bp, 54bp, AA-378bp, 199bp, 54bp.
All enzymes were incubated at 37 degrees for 16 hours. Digested product was analysed in 2% agarose for rs743572, rs9322331 and 2.5% agarose for rs4986938 [Table/Fig-1]. All PCR, enzyme digestion and electrophoresis conditions were standardised in the laboratory.
Gel picture of restriction enzyme digested products of CYP17A (T/C, rs 743572; 414, 290 and 124 bp fragments); (CC-290bp and 124bp, TT-414bp, TC-414bp, 290bp and 124bp, PCR-414bp, Ladder-100bp).
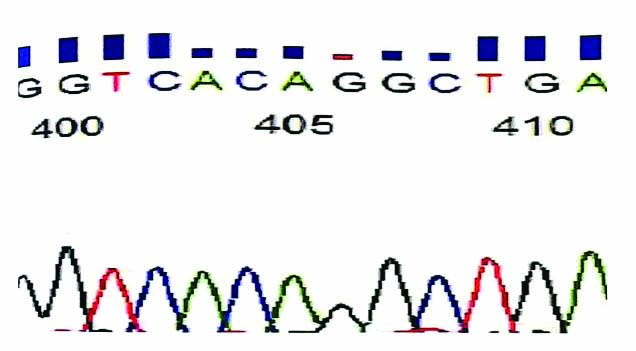
Two samples from each group were sent for sequencing on ABI 3730xl analyser (Applied Biosystems) using software Chromatogram Explorer Lite [Table/Fig-2].
DNA sequencing picture on ABI 3730X1 analyser (Applied Biosystems) using software Chromatogram Explorer Lite.
All chemicals were of molecular biology grade and were obtained from SRL, Sigma and Merck. Molecular work reagents were obtained from Fermentas and Krishgen. Primers were synthesised by Eurofin Bangalore, India.
Statistical Analysis
Graph Pad Prism 6.0 was used. Data is presented as Mean±SEM. Hardy Weinberg equilibrium was tested using Chi-square goodness of fit test. Nominal variables were analysed using Fisher-exact test. The p-value of <0.05 was considered significant.
Results
The average age at menarche was significantly lower in case group (11.51±0.19 year vs 12.04±0.12, p=0.03) showing a longer exposure to sex steroids. Number of previous pregnancies was significantly lower in cases (1.06±0.09 vs 1.41±0.12, p=0.01). However, BMI of cases and controls were comparable (24.45±0.39 vs 23.84±0.38, p=0.27).
The genotype distribution of all subjects studied in ER alpha rs9322331, ER beta rs4986938 and CYP17 rs743572 gene followed Hardy Weinberg equilibrium. There was no significant difference in genotype frequencies between cases and controls (ER alpha rs9322331 C allele (cases vs controls; 62% vs 64%), T allele (38% vs 36%); ER beta rs4986938 G allele (64% vs 59%), A allele (36% vs 41%); CYP17 rs743572 T allele (45.95% vs 47.53%), C allele (55% vs 53%); p-value not significant in any [Table/Fig-3].
The genotype distribution of all subjects studied in ER alpha rs9322331, ER beta rs4986938 and CYP17 rs743572 gene.
| Gene | Restriction site | Allele | Cases (%)N=100 | Controls (%)N=100 | p-value* |
|---|
| ER alpha | rs9322331 | C | 62% | 64% | >0.05 |
| T | 38% | 36% |
| CC | 36% | 38% |
| TC | 52% | 52% |
| TT | 12% | 10% |
| ER beta | rs4986938 | G | 64% | 59% | >0.05 |
| A | 36% | 41% |
| GG | 38% | 35% |
| GA | 52% | 48% |
| AA | 10% | 17% |
| CYP17 | rs743572 | T | 45% | 47% | >0.05 |
| C | 55% | 53% |
| TT | 19% | 23% |
| TC | 51% | 48% |
| CC | 29% | 30% |
*Fisher’s-exact test has been used to analyse between groups.
On analysis of anthropometric parameters with respect to different genotypes of CYP17 rs743572, BMI was significantly higher in women with TC allele of rs743572 of CYP17A gene (24.8±0.42) when compared with women of CC and TT allele (23.4±0.43, 23.3±0.55, respectively) [Table/Fig-4]. The TC genotype of CYP17A was associated with higher BMI. The difference was significant specifically in control population. More of the overweight cases had mixed genotype but the difference was not found to be significant. However, the overweight controls were mostly of TC genotype, suggesting that TC genotype women had more propensity towards obesity.
BMI analysis according to CYP17 (rs743572) gene (TT, CC, TC).
| BMI (kg/m2) | TT (N) | CC (N) | TC (N) | p-value |
|---|
| Mean±SEM | 23.3±0.55 | 23.4±0.43 | 24.8±0.42 | <0.05* |
| BMI cases <25 kg/m2 | 10 | 23 | 33 | >0.05 |
| BMI cases ≥25 kg/m2 | 9 | 6 | 19 |
| BMI controls <25 kg/m2 | 18 | 22 | 22 | <0.05# |
| BMI controls ≥25 kg/m2 | 5 | 8 | 26 |
Table analysed using Fisher’s-exact test statistical tool; *p-value CC vs TC=0.012, TT vs TC=0.017; #p-value CC vs TC=0.01, TT vs TC=0.009; The p-value of <0.05 was considered significant
In ER alpha (CC 24.3±0.46, TT 23.6±0.82, TC 24.1±0.37) and ER beta (GG 24.3±0.48, TT 23.1±0.64, TC 24.2±0.37) genotypes, such relation between allele and anthropometric parameters was not seen (p-value not significant).
Discussion
Uterine leiomyoma is the most common pelvic benign tumour. In India, 20% of the cases of hysterectomy reported by women aged 30-49 years are due to fibroids/cysts [3]. Several studies have shown that the decreasing age of menarche is associated with development of fibroid [20-23]. In this study too, it was seen that the mean age at menarche of cases (11.51±0.19 year) was significantly (p<0.05) lower than controls (12.04±0.12 year). Women with early age at menarche might have a different hormonal milieu leading to increased exposure to estradiol and estrone over the years [24].
Similarly, higher parity is associated with reduced fibroid risk as the overall exposure to oestrogen decreases [6]. In this study, the number of previous pregnancies in cases was found to be significantly higher. Full term pregnancy induces long term change in levels of ovarian hormones and growth factors. Extensive uterine tissue remodelling occurs both during and after full term pregnancy [25].
A number of studies have suggested that fibroids tend to run in families [3,26,27]. Ethnicity plays a significant role in its pathogenesis through genetic regulation of oestrogen and ER action explaining its complicated phenotype [28]. It is not surprising to find that ER alpha polymorphisms have no association in this study group as also seen in an Iranian study [15]. While in other studies, the polymorphisms of ER beta gene polymorphism was found to be significantly associated with oestrogen dependent conditions [29,30]. However, another observer did not find any significant difference [31]. A recent Southern India based study showed high oestrogen levels in “TC” genotype of ER alpha receptor polymorphism [12].
In this study, SNP on rs4986938 in promoter region of oestrogen receptor β gene was assessed and there was no significant difference in genotype frequencies. In both the oestrogen receptors genes in this study, on analysing the various anthropometric parameters with respect to the various genotypes, no significant difference was found.
The untranslated 5’ end of CYP 17 contains a single T (in the A1 allele) to C (in the A2 allele) base substitution at 1931 position which creates an additional Sp-1-type (CCACC box) promoter motif between the initiation of translation and transcription start sites [32]. It concludes that the A2 allele is associated with enhanced transcriptional activity making this allele to be associated with increased susceptibility to oestrogen dependent diseases. In a study, women with the A2/A2 genotype had elevated levels of estrone and dehydroepiandrosterone [33]. CYP17 polymorphism could indeed be an important modifier of the corresponding enzyme activity. However, this study did not find any significant difference in genotype frequencies between cases and controls. Yet an additional finding was observed on anthropometric analysis. BMI was significantly higher in women with TC allele of rs743572 of CYP17 gene. In the pathway of oestrogen biosynthesis, CYP17 catalyses the conversion of 17 hydroxypregnenolone to dihydroepiandrosterone (DHEA). The close relationship between DHEA and obesity partially supported the observed association between CYP17 polymorphism and BMI.
Limitation(s)
This study only included women who attended a Tertiary Care Hospital. However, it could be a possibility that many patients especially in the rural setup or in early stages of disease may visit local government dispensaries. So, this study may not be a true reflection of the population especially when the association between SNP and BMI is concerned. Further community-based studies can be planned to establish the conclusions of this study.
Conclusion(s)
Early age at menarche and less number of previous pregnancies are important risk factors for the fibroid uterus as reconfirmed in this study. The allelic frequencies of all the three SNPs, ER alpha rs9322331, ER beta rs4986938 and CYP17 rs743572 were similar in cases and controls indicating that they do not affect susceptibility to disease in women of Asian Indian ethnicity. However, the association of TC allele of CYP17 SNP with higher BMI needs further analysis. Further analysis may be done to explore the possibility of diet modification and BMI maintenance in females having high risk family history, early menarche and low parity along with TC allele.
*Fisher’s-exact test has been used to analyse between groups.
Table analysed using Fisher’s-exact test statistical tool; *p-value CC vs TC=0.012, TT vs TC=0.017; #p-value CC vs TC=0.01, TT vs TC=0.009; The p-value of <0.05 was considered significant
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: May 22, 2020
Manual Googling: Sep 04, 2020
iThenticate Software: Jan 16, 2021 (18%)
[1]. Schwartz SM, Epidemiology of uterine leiomyomataClin Obst Gynaecol 2001 44:316-26.10.1097/00003081-200106000-0001811344995 [Google Scholar] [CrossRef] [PubMed]
[2]. Flake GP, Anderson J, Dixon D, Etiology and pathogenesis of leiomyomas: A reviewEnviron Health Perspect 2003 111:1037-54.10.1289/ehp.578712826476 [Google Scholar] [CrossRef] [PubMed]
[3]. Shekhar C, Paswan B, Singh A, Prevalence, sociodemographic determinants and self-reported reasons for hysterectomy in IndiaReprod Health 2019 16(1):11810.1186/s12978-019-0780-z31375139 [Google Scholar] [CrossRef] [PubMed]
[4]. Benassayag C, Leroy MJ, Rigourd V, Robert B, Honoré JC, Mignot TM, Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: Pregnancy and leiomyomaAm J Physiol 1999 276(6):E1112-18.10.1152/ajpendo.1999.276.6.E111210362625 [Google Scholar] [CrossRef] [PubMed]
[5]. Nierth-Simpson EN, Martin MM, Chiang TC, Melnik LI, Rhodes LV, Muir SE, Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: Implications for proliferationEndocrinology 2009 150(5):2436-45.10.1210/en.2008-022419179429 [Google Scholar] [CrossRef] [PubMed]
[6]. Okolo S, Incidence, aetiology and epidemiology of uterine fibroidsBest Pract Res Clin Obstet Gynaecol 2008 22:571-88.10.1016/j.bpobgyn.2008.04.00218534913 [Google Scholar] [CrossRef] [PubMed]
[7]. Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A, Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implicationsReprod Sci 2017 24(9):1235-44.10.1177/193371911667868627872195 [Google Scholar] [CrossRef] [PubMed]
[8]. Matsuzaki S, Fukaya T, Uehara S, Murakami T, Sasano H, Yajima A, Characterization of messenger RNA expression of estrogen receptor-alpha and -beta in patients with ovarian endometriosisFertil Steril 2000 73:1219-25.10.1016/S0015-0282(00)00527-6 [Google Scholar] [CrossRef]
[9]. Jakimiuk AJ, Bogusiewicz M, Tarkowski R, Dziduch P, Adamiak A, Wrobel A, Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal womenFertil Steril 2004 82:1244-49.10.1016/j.fertnstert.2004.02.13015474102 [Google Scholar] [CrossRef] [PubMed]
[10]. Wang H, Wu X, Englund K, Blanck A, Lindblom B, Sahlin M, Different expression of estrogen receptors α and β in human myometrium and leiomyoma during the proliferative phase of the menstrual cycle and after GnRHa treatmentGynaecol Endocrinol 2001 15:443-52.10.1080/gye.15.6.443.452 [Google Scholar] [CrossRef]
[11]. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T, Progesterone is essential for maintenance and growth of uterine leiomyomaEndocrin 2010 151(6):2433-42.10.1210/en.2009-122520375184 [Google Scholar] [CrossRef] [PubMed]
[12]. Govindan S, Shaika NA, Vedicherlaa B, Kodatia V, Raoc KP, Hasana Q, Estrogen receptor alpha gene (T/C) Pvu polymorphism in endometriosis and uterine fibroidsDisease Markers 2009 26:149-54.10.1155/2009/58026019729795 [Google Scholar] [CrossRef] [PubMed]
[13]. Neuss K, Elling D, Cascorbi I, Lueftner D, Association between breast cancer and estrogen receptor gene polymorphism PVU IIJ Clin Oncol 2006 24:64310.1200/jco.2006.24.18_suppl.643 [Google Scholar] [CrossRef]
[14]. An S, Li E, Tong X, Study on relationship between estrogen receptor gene polymorphism and syndrome differentiation typing of female postmenopausal osteoporosis in Traditional Chinese medicineZhongguo Zhong Xi Yi Jie He Zha Zhi 2000 20(12):907-10. [Google Scholar]
[15]. Taghizade MF, Tagatabaiefar MA, Hashemzadeh CM, Miraj S, Lack of association between ESR1 and CYP17A1 gene polymorphisms and susceptibility to uterine leiomyoma in female patients of Iranian descentCell J 2014 16(2):225-30. [Google Scholar]
[16]. Lamp M, Peters M, Reinmaa E, Haller-Kikkatalo K, Kaart T, Kadastik U, Polymorphisms in ESR1, ESR2 and HSD17B1 genes are associated with fertility status in endometriosisGynaecol Endocrinol 2011 27(6):425-33.10.3109/09513590.2010.49543420586553 [Google Scholar] [CrossRef] [PubMed]
[17]. Amant F, Dorfling CM, De Brabanter J, Vandewalle J, Vergote I, Lindeque BG, A possible role of the cytochrome P450c17 alpha gene (CYP17) polymorphism in the pathobiology of uterine leiomyomas from black South African women: A pilot studyActa Obs et Gynaec Scandin 2004 83(3):22-26.10.1111/j.1600-0412.2004.00422.x14995917 [Google Scholar] [CrossRef] [PubMed]
[18]. Integrated DNA technologies. Oligoanalyser tool. Available from https://www.idtdna.com/pages/tools/oligoanalyser. (Latest accessed on 28/8/2020) [Google Scholar]
[19]. Free online lab tools. Neb cutter V2.0. Available from http://www.labtools.us/nebcutter-v2-0/a. (Latest accessed on 28/8/2020) [Google Scholar]
[20]. Velez Edwards DR, Baird DD, Hartmann KE, Association of age at menarche with increasing number of fibroids in a cohort of women who underwent standardised ultrasound assessmentAm J Epidem 2013 178(3):426-33.10.1093/aje/kws58523817917 [Google Scholar] [CrossRef] [PubMed]
[21]. Wise LA, Laughlin-Tommaso SK, Epidemiology of uterine fibroids: From menarche to menopauseClin Obstet Gynaecol 2016 59(1):2-24.10.1097/GRF.000000000000016426744813 [Google Scholar] [CrossRef] [PubMed]
[22]. Ciavattini A, Di Giuseppe J, Stortoni P, Montik N, Giannubilo SR, Litta P, Uterine fibroids: Pathogenesis and interactions with endometrium and endomyometrial junctionObstet Gynaecol Int 2013 2013:17318410.1155/2013/17318424163697 [Google Scholar] [CrossRef] [PubMed]
[23]. Sarkodie BD, Botwe BO, Adjei DN, Ofori E, Factors associated with uterine fibroid in Ghanaian women undergoing pelvic scans with suspected uterine fibroidFertil Res Pract 2016 2:910.1186/s40738-016-0022-928620536 [Google Scholar] [CrossRef] [PubMed]
[24]. Emaus A, Espetvedt S, Veierød MB, Ballard-Barbash R, Furberg AS, Ellison PT, 2007. 17-β-estradiol in relation to age at menarche at menarche and adult obesity in premenopausal womenHuman Reproduction 2008 23(4):919-27.10.1093/humrep/dem43218227106 [Google Scholar] [CrossRef] [PubMed]
[25]. Walker CL, Cessen-Cummings K, Houle C, Baird D, Barrett JC, Davis B, Protective effect of pregnancy for development of uterine leiomyomaCarcinogenesis 2001 22:2049-52.10.1093/carcin/22.12.204911751438 [Google Scholar] [CrossRef] [PubMed]
[26]. Payson M, Leppert P, Segars J, Epidemiology of myomasObstet Gynaecol Clin North Am 2006 33(1):01-11.10.1016/j.ogc.2005.12.004 [Google Scholar] [CrossRef]
[27]. Simms-Stewart D, Fletcher H, Counselling patients with uterine fibroids: A review of the management and complicationsObstet Gynaecol Int 2012 2012:53936510.1155/2012/53936522272207 [Google Scholar] [CrossRef] [PubMed]
[28]. Hsieh YY, Chang CC, Tsai FJ, Lin CC, Tsai CH, Estrogen receptor alpha dinucleotide repeat and cytochrome P450c17alpha gene polymorphisms are associated with susceptibility to endometriosisFertil Steril 2005 83(3):567-72.10.1016/j.fertnstert.2004.07.97715749482 [Google Scholar] [CrossRef] [PubMed]
[29]. Ashton KA, Proietto A, Otton G, Symonds I, McEvoy M, Attia J, Estrogen receptor polymorphisms and the risk of endometrial cancerBrit Jour Obstret Gynaecol 2009 116(8):1053-61.10.1111/j.1471-0528.2009.02185.x19438492 [Google Scholar] [CrossRef] [PubMed]
[30]. Treeck O, Elemenler E, Kriener C, Horn F, Springwald A, Hartmann A, Polymorphisms in the promoter region of ESR2 gene and breast cancer susceptibilityJ Steroid Biochem Mol Biol 2009 114(3-5):207-11.10.1016/j.jsbmb.2009.02.01219429453 [Google Scholar] [CrossRef] [PubMed]
[31]. Levens ED, Wesley R, Premkumar A, Blocker W, Nieman LK, Magnetic resonance imaging and transvaginal ultrasound for determining fibroid burden: Implications for research and clinical careAm J Obst Gynaecol 2009 200(5):25710.1016/j.ajog.2008.12.03719268886 [Google Scholar] [CrossRef] [PubMed]
[32]. Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Steroid metabolism gene CYP17 polymorphism and the development of breast cancerCancer Epidemiol Biomarkers Prev 2000 9(12):1343-48. [Google Scholar]
[33]. Haiman CA, Hankinson SE, Colditz GA, Hunter DJ, De Vivo I, A polymorphism in CYP17 and endometrial cancer riskCancer Res 2001 61(10):3955-60. [Google Scholar]