In the last decade, research has focused on the potentially hazardous effects of a wide range of chemicals which are present in the human or wildlife environment. Exogenous chemical substances having potential to alter the endocrine system integrity with subsequent adverse health effects on the organisms are called Endocrine Disruptors (EDs) [1]. Nonylphenol Polyethoxylates (NPEs), which are highly cost-effective surfactants with exceptional performance, are widely used in institutional, commercial and industrial applications. NPEs are frequently applied in pesticides, detergents, insecticides and other synthetic products [2]. These NPEs get biodegraded into four by-products: 4-NP, nonylphenol diethoxylate, nonylphenol triehoxylates and nonlyphenoxyacetic acid [3]. In these byproducts, NP is widely used worldwide, and its large amount is discharged into the ecosystem, especially into water. 4-NP, which is used in surfactants or plastic, petroleum processing, and phthalate esters, are used as plasticisers in polyvinylchloride applications and nitrocellulose [4].
Thus, people get exposed to this chemical and are unaware of its potential risks. NP is a well-known EDC and it acts by mimicking the natural hormone 17-β-estradiol and tends to compete for oestrogen receptor binding sites [1]. A 17-β-estradiol hormone influences the development and maintenance of male and female sex characteristics [5]. Environmental oestrogens have a weak oestrogenic activity while the activity of NP is approximately 10-3 to 10-6 times less oestrogenic than estradiol [6]. Recently, it was found that NP also has anti-androgenic activity and can disturb the proper functioning of androgens. As androgens are essential for normal development, including that of the reproductive systems in males, NP may induce male reproductive toxicity by disturbing the function of endogenous oestrogens via receptor mechanism and also causes the cell death by modulating cellular mechanism via its phenolic group [7]. A set of biomarkers is generally used to evaluate the biological effects of pollutants, and these are an early warning for detrimental biological effects.
Cortisol is a hormone which is mainly released at the time of stress and increases blood pressure to distribute the glucose and other nutrients to the cells [8]. Thus, it helps the body to resist stress and reduces inflammatory responses. The quantification of cortisol, testosterone and estradiol hormones was performed directly from aliquots of the medium by Enzyme-Linked Immunosorbent Assay (ELISA), and these can be studied as a marker for endocrine effects caused by any external or internal factor. A recent study shows that Bisphenol-A (BPA) is also an oestrogenic EDCs like NP. It is used in several plastic and consumer products. BPA alters oxidative stress parameters by increasing Malondialdehyde (MDA) and Reactive Oxygen Species (ROS) levels, and decreasing SOD activities, in BPA-treated rats as compared to control [9]. A study on aquatic bodies results the same that due to exposure to EDC’s, they undergo oxidative stress and respond by changing in activity of the antioxidant enzymes and morphological alterations in liver and kidney tissues [10]. A detailed study of different factors and tissue architecture of organs in NP exposed mice in comparison to control, will help to stablish, oxidative stress and histopathological biomarkers for causative effects of NP. This study aimed to examine the multigenerational effects of 4-NP on reproductive, histological and hormonal fitness of male mice exposed to the chemical for 21 days.
Materials and Methods
This toxicological study was performed on mice model, at the Department of Zoology, Institute of Science, in collaboration with Department of Anatomy, Institute of Medical Science, Banaras Hindu University, Varanasi, Uttar Pradesh, India. The study period was from January to August 2020. Ethical clearance was obtained from the Institutional Ethical Committee of institution for this study (Reference No: Dean/2020/MCE/2033 Dated15.07.2020).
Total 30 adult male mice (25-30 g and 70-80 days old) of BALB/c strain were selected to conduct the experiment. Animals were maintained according to the guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India (CPCSEA, 2003).
Inclusion criteria: Male mice each of 25-30 g and 70-80 day-old of BALB/c strain were included in the study.
Exclusion criteria: Female mice were not included in this study. While male mice <25 g or >25-30 g (underweight or overweight) and younger than 70-80 days were excluded from the study, as the reproductive organs may not be developed.
Chemicals and reagents: 4-NP (CAS register no: 84852-15-3, 99% purity, Molecular weight was 220.35, Density was 0.940) mixture of isomers was purchased from Acros Organics. It is soluble in water at 25°C and the solubility of NP is 7 mg/L or 0.007 mg/mL. The LD50 of 4-NP is 170 mg/kg BW [11]. LD50 is the amount/dose of a substance/chemical (usually per BW that causes death/mortality of 50% of the test animals). The two doses for the experiment were designed based on LD50 of NP and were continued for 21 days.
Animal collection and acclimatisation: Mice were maintained under well hygienic conditions in well ventilated room at 25-28°C with 12 hours photoperiodic (8 am-8 pm) and were provided with pellet food and drinking water. Animals were divided into control group, and experimental group of 21.25 mg/kg BW (1/8th of LD50), and 85 mg/kg BW (1/2th of LD50) dose, each having 10 male mice and all groups were kept for seven days for acclimatisation.
Nonylphenol (NP) was dissolved in water and administered by oral gavage to all mice of experimental groups in continuation for 21 days. The two doses were selected on the basis of very low to high exposure of NP that can be expected to be in the environmental scenario [11].
Tissue and blood collection: After 21 days of drug administration, mice were first anaesthetised and sacrificed by decapitation to collect blood. Before sacrificing the mice, the weights were measured. The weights of testis, epididymis, liver, kidney and brain tissues were taken. These tissues were kept in bouin’s and para formaldehyde solution for histological studies and a part of these tissues were kept at -20°C for further uses.
Sperm concentration and viability: Assessment of sperm concentration was done in control and experimental groups. First, mice epididymis was removed, washed in 1X Phosphate Buffer Solution (PBS) and then chopped-off in 1 mL of 1X PBS. Further, it was diluted 20 times in 1X PBS and one drop of it was taken on haemocytometer and sperms number were counted and evaluated.
For sperm viability test one drop of diluted sample with one drop of eosin and one drop of nigrosin stain was taken on a clean glass slide, mixed and then a uniform smear was made. Slide was left for air dry, mounted and then observed under the microscope. The dye exclusion technique was performed to distinguish live and immotile spermatozoa from dead spermatozoa.
Profiling of hormonal parameters: Blood was taken in micro centrifuge tube, labelled, kept at room temperature for four hours until the serum had been separated out. Then the blood samples were centrifuged at 4200 rpm for seven minutes at 4°C. Then serum was separated out and stored at -20°C for further use in hormonal assay. The DRG Cortisol Enzyme-Linked Immunosorbent Assay (ELISA) kit was used for cortisol measurement and its absorbance was taken at 450 nm. Diametra Testosterone kit was used for the measurement of concentration of total testosterone (free+ bound) and its absorbance was measured at 450 nm. The measurement of concentration of estradiol was done by Diametra Estradiol kit and its absorbance was measured at 450 nm.
Histopathological studies: Initially tissue (testes and liver) were taken and stored into Bouin’s solution, dehydrated and blocks were prepared. Tissue sections were sliced into 6 μm using the rotator microtome (Model Leica RM 2245). Slides were processed for H&E for testes and liver histological studies. PAS staining was performed in liver sections for its tissue architecture. IHC of Caspase-3 antibody was performed in liver tissues to see the effect of 4-NP on apoptotic pathway.
Determination of oxidative stress parameters: To determine the effect of treatment caused by oxidative stress in brain, liver, kidney and testis, the antioxidant enzymes SOD and LPO assay was performed. A 10% tissue homogenate was prepared in ice cold phosphate buffer solution (PBS, pH-7.4) using homogeniser (IKA T10 basic, ULTRA-TURRAX). Tissue homogenate was centrifuged at 12000 g for 30 minutes at 4°C to obtain the Post Mitochondrial Fraction (PMF) which was further used for SOD and Thiobarbituric Acid Reactive Substances (TBRAS) levels. The level of SOD [12] and LPO [13] were estimated by the standard protocols.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software, version 23.0. One-way Analysis of Variance (ANOVA) test was performed for the statistical significance of the differences in mean values of variables among several groups. The data were presented as Mean±SD (Standard Deviation) and means were separated using Tukey’s test, when data were normally distributed, and variances were homogeneous. The p-values <0.05 were considered statistically significant.
Results
Results showed that the respective weights of brain, kidney and liver tissues in the two doses, in respect of control were significantly higher (p≤0.005). The BW, testes and Seminal Vesicle (SV) weights in respect to control group were not significantly different. Profiling of hormones (Cortisol and Testosterone) in serum of 4-NP treated groups of mice were significantly lower while for Estradiol was higher, in comparison to control respectively. The values of SOD in brain, kidney, testes and liver tissues for two doses were significantly lower in comparison of control. While LPO in respective organs for two dose groups were significantly higher (p<0.005) in comparison to control group, showing the adverse effects of 4-NP on vital organs of mice, administrated a specific dose of NP for 21 days.
[Table/Fig-1] shows that NP administration to adult male mice for 21 days did not cause any significant change in the BW of the mice when compared to the control groups. But both doses (21.25 mg/kg BW and 85.0 mg/kg BW) statistically increased the absolute kidney and liver weights.
Body and organ weight of control and experimental groups.
| Exposure | Control (N=10) | 21.25 mg/kg BW (1/8th of LD50) (N=10) | 85 mg/kg BW (1/2th of LD50) (N=10) | p-value (21.25 mg/kg BW vs Control, 85 mg/kg BW vs Control) |
|---|
| Body weight (g) | 31.66±0.33 | 30.66±2.96 | 32.0±1.52 | 0.302, 0.498 |
| Brain (mg) | 423.0±6.67 | 416.0±3.3 | 423±3.33 | 0.008*, 1.00 |
| Kidney (mg) | 463±12.03 | 480.0±23.12 | 482±9.60 | 0.053*, 0.001* |
| Liver (g) | 1.42±0.08 | 1.48±0.085 | 1.54±0.034 | 0.121, <0.001** |
| Testis (mg) | 166±7.27 | 158±24.42 | 173.0±13.34 | 0.333, 0.162 |
| Seminal vesical (mg) | 206.0±13.34 | 211±4.41 | 213±3.33 | 0.275, 0.124 |
Independent student’s t-test was used to compare the mean for the NP treated and control group. Two-tailed p-values less than 0.05 was considered statistically significant. Results are presented as mean±SD for 10 males per experimental group
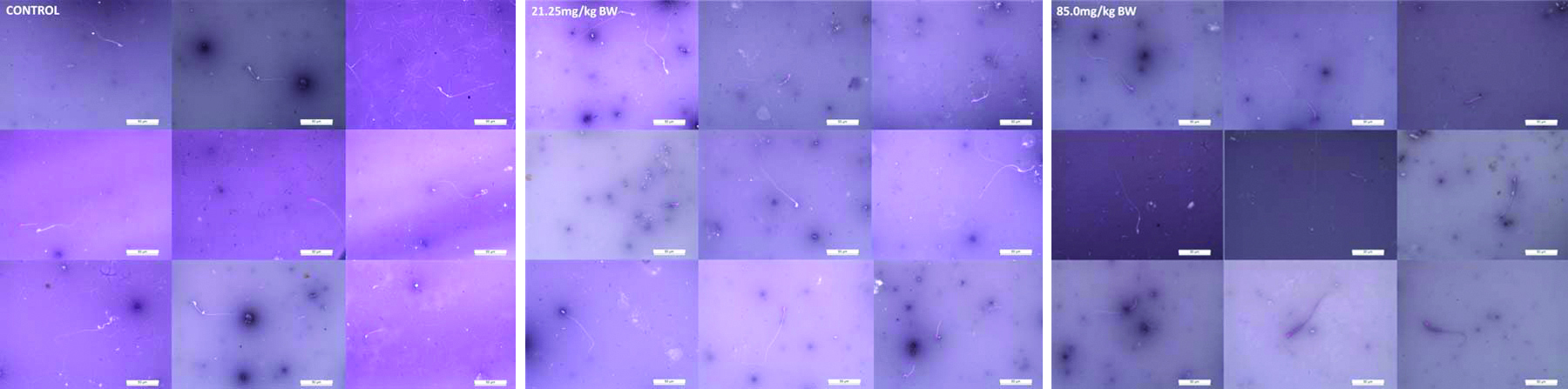
Photomicrographs show the viable and dead sperms in mice of control and experimental groups. The viable or live sperms are colourless (transparent) while dead sperms take the colour of the stain and are seen as purple because of the distortion of their plasma membrane. The control group showed mostly viable and very few dead sperms while experimental groups showed more of dead and lesser viable sperms comparatively in low and high doses of 4-NP. The sample (~75 μL, 20 times diluted in 1X PBS) was taken on slides, observed under the microscope for their viability and counting was done per section on haemocytometer [Table/Fig-2].
Sperm concentration and viability assessment (Eosin and Nigrosin Stain, 40X).
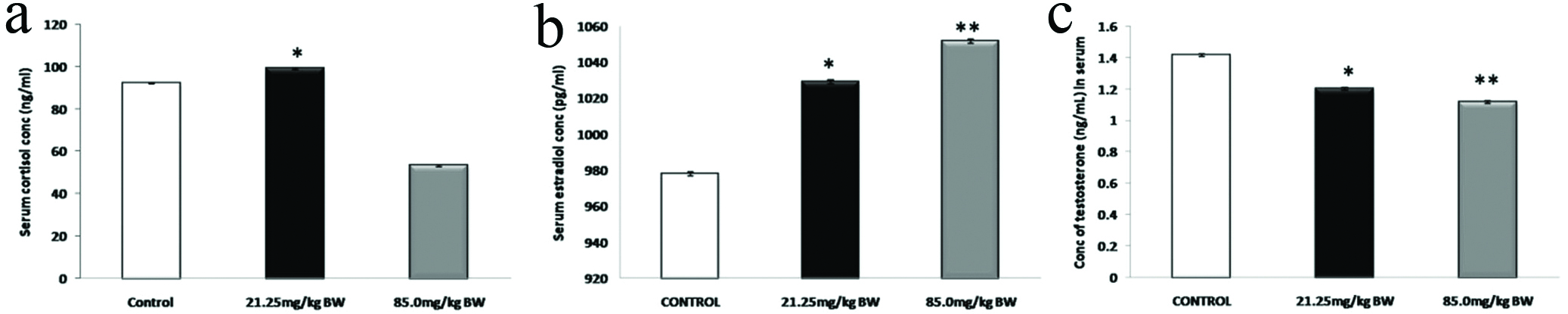
[Table/Fig-3] shows the reduced levels of cortisol hormone (a) in 85.0 mg/kg BW and increased level in 21.25 mg/kg BW experimental group. The average level of testosterone (b) showed significant reduction in the experimental groups compared to control groups. The mean value of Estradiol, 17β-estradiol (c) showed a significant increase in the experimental groups as compared to control groups. The male mice treated with 4-NP showed dose-dependent increase for Estradiol hormone, in the serum.
Hormone profiling of cortisol, estradiol and testosterone in serum.
*p<0.05, **p<0.01
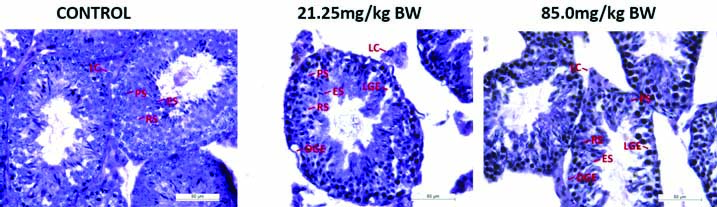
[Table/Fig-4] shows control group had Pachytene Spermatocytes (PS), Round Spermatid (RS) and Elongated Spermatid (ES) and well defined Leydig Cells (LC). Testicular sections of 4-NP treated mice at two doses showed some adverse effects. At few places germinal epithelium was found to be degenerated (DGE). With this Loosening of Germinal Epithelium (LGE) were also seen at few places. In seminiferous tubules Sloughing of Germinal Epithelium (SGE) were also seen in testicular sections of mice treated with 4-NP.
Photomicrographs of transverse section of testes of mice of control and experimental groups to study of histological changes in tissue architecture by (H&E, 40X).
[Table/Fig-5] shows liver section from mice in control group (a and d) showed normal liver histology. Differences in the experimental groups (21.25 mg/kg BW and 85.0 mg/kg BW) were observed with respect to the control. Treated groups showed mixed dilated portal vein, lympho-monocytic infiltrations around the portal vein in both low dose (b and e) and high dose (c and f) with mild sinusoid.
Photomicrographs of transverse section of liver of mice of control and experimental groups (H&E, a-c 10X and d-f 40X).

[Table/Fig-6] Showed glycogen deposits were observed in the hepatocyte cytoplasm (A), and these accumulations were seen as pink areas of PAS-positive material throughout the section in control with Portal Vein (PV), nucleus of hepatocyte (B) and sinusoids (C). While glycogen deposits were seen lesser with more sinusoidal space in 21.25 mg/kg BW (b and e) and 85.0 mg/kg BW (c and f) experimental groups as compared to control group (a and d).
Photomicrographs of transverse section of the mice liver of control and experimental groups (LD and HD) for 21 days doses by Periodic Acid Schiff stain (PAS, a-c 10X and d-f 40X).

[Table/Fig-7] shows a dose-dependent elevation in the level of Caspase-3, which represents larger sub-unit of activated caspase-3, are observed following 4-NP administration (21.25 mg/kg BW and 85.0 mg/kg BW) for 21 days. IHC staining of active caspase-3 in mice liver is localised in cytoplasm of hepatocytes. Expression of caspase-3 was significantly increased at the dose levels of 21.25 mg/kg BW (b and e) and 85.0 mg/kg BW (c and f) as compared to control group (a and d).
Immunostaining of Caspase-3 in mice liver tissues of control and experimental groups (IHC, a-c 10X and d-f 40X).

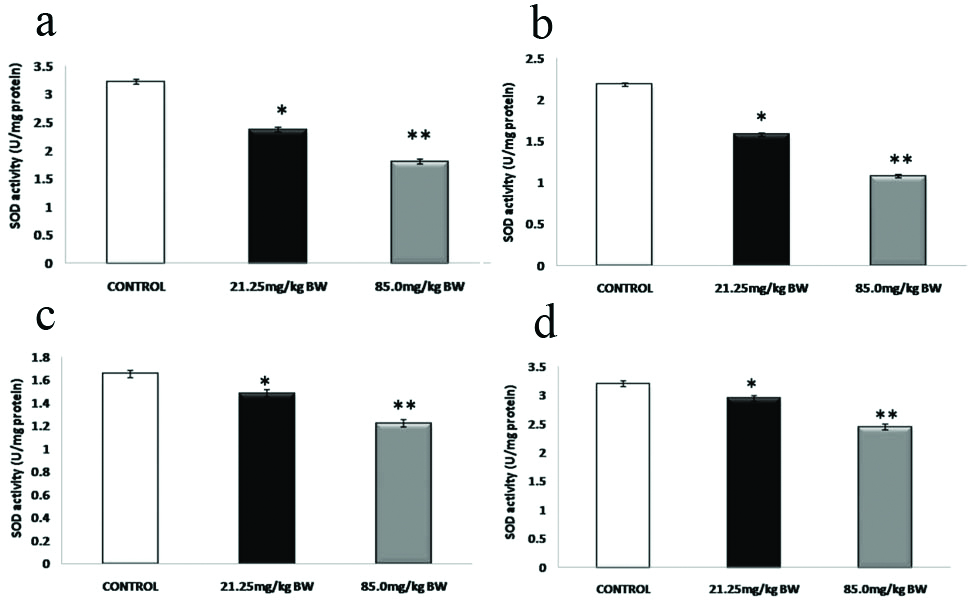
[Table/Fig-8] showed the effect of 4-NP on the activity of SOD in the brain (a), kidney (b), liver (c) and testes (d) of the male mice in experimental groups with respect to control (Mean±SD). 4-NP administration at 21.25 mg/kg BW and 85.0 mg/kg BW for 21 days caused a decreased in the activities of SOD in all four tissues (brain, kidney, liver and testis) of male mice.
Biochemical assay of SOD in brain, kidney, liver and testes tissue samples.
p>0.05, *p<0.05, **p<0.01
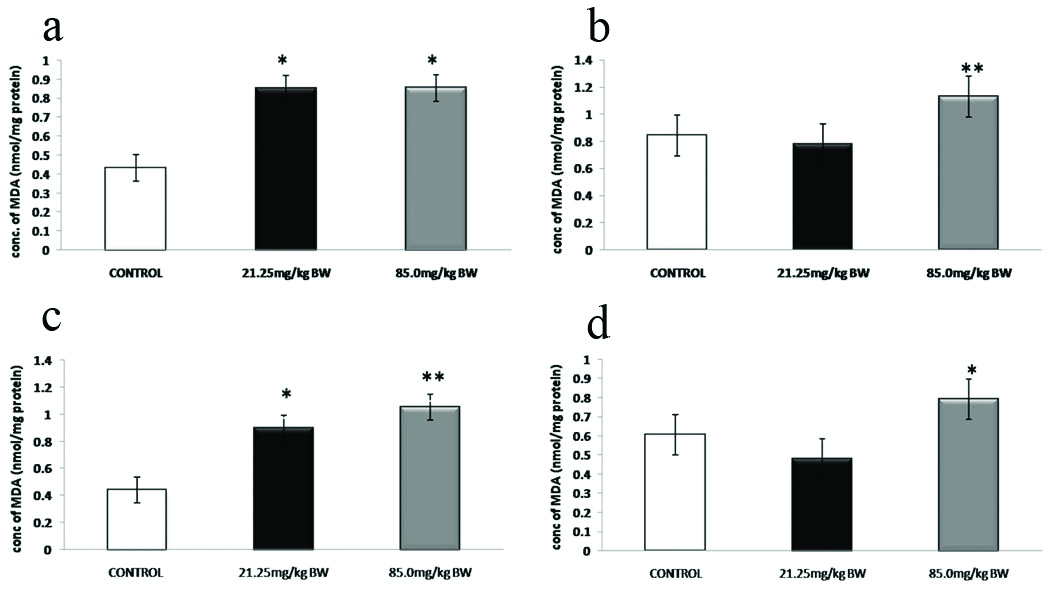
[Table/Fig-9] showed the effect of 4-NP on the levels of LPO in the brain (a), kidney (b), liver (c) and testes (d) of adult male mice in experimental groups with respect to control (Mean±SD). In both 4-NP-treated groups 21.25 mg/kg BW and 85.0 mg/kg BW an increase in the levels of LPO were observed in all four, brain (a), kidney (b), liver (c) and testes tissue samples (d) as compared to the control group.
Biochemical assay of Lipid Peroxidation (LPO) in brain, kidney, liver and testes tissue samples.
p>0.05, *p<0.05, **p<0.01
Discussion
In the present study, 4-NP was administered to mice by oral gavage, as humans are primarily exposed to the compound through their diet. The experimental study showed the histopathological architecture changes in testes and liver, variation in hormones (Cortisol, Testosterone and Estradiol) involved in reproduction and oxidative stress parameters (SOD and LPO), that developed due to 4-NP, on male mice exposed to the chemical for 21days.
Laboratory studies on animals have demonstrated adverse effects of 4-NP, such as on reproduction, development, neurotoxicity and inflammation [14,15]. By staining procedure (E-N stain) sperms were evaluated for their viability. In a previous study, it has been shown that counting and viability of sperms has been diminished after an exposure to EDC’s similar to this study [7]. Similar distortion in architecture of liver and testes of mice has been observed, as has been observed previously by an exposure to EDC’s by other organisms including mice [14,16].
Lipid peroxides, a marker of oxidative stress, are also known to be increased in liver and other organs as a result of oxidative stress [17]. It has been reported that MDA, a highly reactive by-product of LPO could react with DNA bases or could produce bifunctional intermediates leading to the formation of exocyclic DNA adducts [18]. It is therefore possible that elevated levels of LPO, may be a cause for DNA damages in mice liver and other body organs via formation of adducts, following 4-NP exposure for 21days. Apoptosis or programmed cell death is an important process involved in normal development and maintenance of cellular homeostasis. Over-stimulation of apoptosis in liver can lead to significant hepatocellular damages [19]. Increased level of ROS has been reported to initiate apoptosis pathway.
In this study, the expression of apoptotic protein (Caspase-3), was found to be higher as compared to normal control liver tissue of mice. Caspase-3 expression together with evaluation of ROS (SOD and LPO) elucidate the toxic effect of 4-NP. The mean values of reproductive hormones and stress parameters ROS (SOD and LPO) together with expression of apoptotic protein (Caspase-3), has not be studied previously.
Administration of 4-NP doses caused stress in mice that has been shown by elevated cortisol in experimental groups. The results of the study showed that 4-NP has adverse effects by decreasing testosterone and increasing estradiol in experimental group as compared to control group.
Limitation(s)
This study did not include the effect of 4-NP on female mice, on its reproduction, physiology or hormonal imbalance. The second limitation of this study was the lack of cellular and histological experiments, which are not focused to find out the molecular mechanism behind it.
Conclusion(s)
Thus, it can be concluded that the toxicological effects exerted by 4-NP occur probably by affecting the endocrine system and/or changes in other hormonal function. 4-NP is an ED that has an adverse effect on the health of the organisms. It can damage reproductive systems in species and these damages are complicated via its mechanisms. Hormonal disturbance might be one of these mechanisms and by considering the role of hormonal homeostasis in reproduction, one can conclude that these toxins exert their adverse effects on the reproduction and physiological processes via disturbing the hormonal homeostasis.
Authors’ contributions: KK has drafted the research article and AA has helped in revising it critically for intellectual content. KK has helped in design and drafting of article and AA has helped in statistical analysis of the data. RC and RS have given feedback, suggestions and final approval for the article.
Financial support and sponsorship: This research work was supported by CSIR-Senior Research Fellowship awarded to Ms. Kusum and in part by grants from UGC-UPE and UGC-CAS, New Delhi, India.
Independent student’s t-test was used to compare the mean for the NP treated and control group. Two-tailed p-values less than 0.05 was considered statistically significant. Results are presented as mean±SD for 10 males per experimental group