Breast cancer is the most common cancer in women, with a global incidence of about 2.1 million cases each year, and in India, it is 1,62,468 per year [1]. Although core biopsy is considered standard, FNAC is widely practiced, as it’s a rapid, minimally invasive, accurate, cost-effective technique that is well accepted by patients. Core biopsy/FNAC is considered a valuable tool in the preoperative assessment of breast lesions [2]. FNAC use in the evaluation of breast lesions has changed substantially since the 1996 National Cancer Institute consensus meeting regarding breast FNAC reporting, mainly due to changes in screening programs, available treatments, and a recent preference for a Core Needle Biopsy (CNB) in some settings [3,4]. In 2016, the International Academy of Cytology (IAC) established a "Breast Group" composed of pathologists, radiologists, surgeons, and oncologists to produce comprehensive and standardised guidelines for breast FNAC reporting [2,5]. The IAC Breast Group met together in 2016 at the Yokohama International Congress of Cytology, intending to develop an internationally recognised and standardised reporting system that would define best practice guidelines for the use of FNAC in diagnosing breast lesions more consistently and accurately [2,5]. The System has established uniform terminology for five defined categories, (1-5, Category 1-Insufficient material, Category 2-Benign, Category 3-Atypical, Category 4-Suspicious of malignancy, Category 5-Malignant) for breast FNAC with stratified associated Risk Of Malignancy (ROM) and management recommendations [2,5,6]. This System stresses on writing the name of the category, with category number, rather than just the category number. The study’s main objective is to categorise breast FNACs according to the IAC Yokohama system and correlate with histopathology. This eventually will have uniformity in reporting the FNACs among the fellow cytopathologist. This study also helps to calculate the ROM, which further helps the clinician manage the patients accordingly.
Materials and Methods
The present retrospective cohort study was conducted at KMIO, Bangalore from January 2017-December 2018. Study included 1,467 breast FNAC cases. All new clinically and radiologically investigated cases of breast lesions were included and recurrent previously treated cases of carcinoma breast were excluded from the present study. Since the study is retrospective in design and did not involve any intervention, an exemption from Ethical Committee was taken. A broad consent was taken for patients clinical details and procedures. Sample size calculation was done using the formula, when proportion used is a qualitative variable [7,8]. An article by Montezuma D et al., was considered to derive sample, in their study to assess the ROM, 21% of cases had histopathology diagnosis with 10% precision and a 95% confidence interval [8]. The minimal sample size required is 1445, using the formula, with proportion (p) of 21%=0.21, relative precision (d) of 10%, and α value of 5%, and hence 1,467 cases were included in the present study [7,8].
FNAC for palpable breast lumps were done by routine FNAC and non-palpable lesions were done by ultrasound guidance. FNAC was performed using a 23-24-gauge needle. A total of 2 to 4 smears were prepared and were air-dried and wet fixed using in-house prepared Polyethylene Gycol (PEG). Air-dried smears were stained by May Grunwald-Giemsa (MGG) stain and wet fixed smears were stained by Papanicolaou stain. Corresponding histopathological diagnosis was obtained and cyto-histopathology diagnosis was compared and analysed. ROM was calculated by dividing confirmed malignant cases in each category to the total number of cases in the diagnostic category.
Breast FNAC’s done, were retrieved and were reclassified based on the newly proposed IAC Yokohama System, into five categories (Category 1-Insufficient material, Category 2 -Benign, Category 3-Atypical, Category 4-Suspicious of malignancy, Category 5-Malignant) and was compared with histopathology, ROM was assessed whenever possible [2]. Comparison of the FNAC interpretation was done [Table/Fig-1].
Comparison of the FNAC interpretation [2].
| Earlier interpretation | Interpretation based on new system of classification-IAC Yokohama system |
|---|
| No significant material. | Category1 : Insufficient material |
| Benign breast disease (Fibroadenoma) followed by cystic lesions. | Category 2 : Benign |
| Atypical cells | Category 3 : Atypical |
| Suspicious of malignancy | Category 4 : Suspicious of malignancy |
| Primary metastatic | Category 5 : Malignant |
Statistical Analysis
The study results were analysed using Microsoft excel 2007 and sensitivity, specificity, PPV, and NPV, and accuracy ratios were calculated using the MedCalc diagnostic test evaluation calculator, keeping histologic diagnosis as the gold standard.
Results
Total of 1,467 FNACs were done. Age ranged from 11-83 years with mean age of 48 years. Male to female ratio was 1:53.33 (n, males=27, females=1440). Categorisation of lesions using FNAC is summarised in [Table/Fig-2].
Categorisation of FNAC lesions based on IAC Yokohama system.
| Category | Parameters | No. of cases (n/%) |
|---|
| Category 1 | Insufficient material | 100/07 |
| Category 2 | Benign | 356/24 |
| Category 3 | Atypical | 106/07 |
| Category 4 | Suspicious of malignancy | 047/03 |
| Category 5 | Malignant | 858/59 |
| Total | 1467 |
Category 1 (Insufficient) were the cases with sparse cellularity or too poorly smeared or fixed to allow a cytomorphological diagnosis. These cases were earlier called NSM (No Significant Material).
Smears showing features of fibroadenoma and other benign breast diseases were placed in Category 2 (Benign) [Table/Fig-3a,b]. A benign breast FNAC diagnosis is made in cases that have unequivocally benign cytological features, which may or may not be diagnostic of a specific benign lesion. This includes cases like the most common benign lesions diagnosed by FNAC like acute mastitis and breast abscess, granulomatous mastitis, foreign body reactions such as silicone, fat necrosis, fibrocystic change, lactational change, fibroadenoma, gynecomastia in males.
a) Photomicrograph cytology of fibroadenoma (Pap, 4x); b) Photomicrograph cytology of fibroadenoma (MGG, 4x): c) Photomicrograph cytology of atypical cell clusters (Pap, 10x); d) Photomicrograph cytology of atypical cell clusters (MGG, 10x); e) Photomicrograph cytology of suspicious cell cluster (Pap, 40x); f) Photomicrograph cytology of suspicious cell cluster. (MGG, 40x); g) Photomicrograph cytology of duct carcinoma (Pap, 40x.); h) Photomicrograph cytology of duct carcinoma (MGG, 40x).
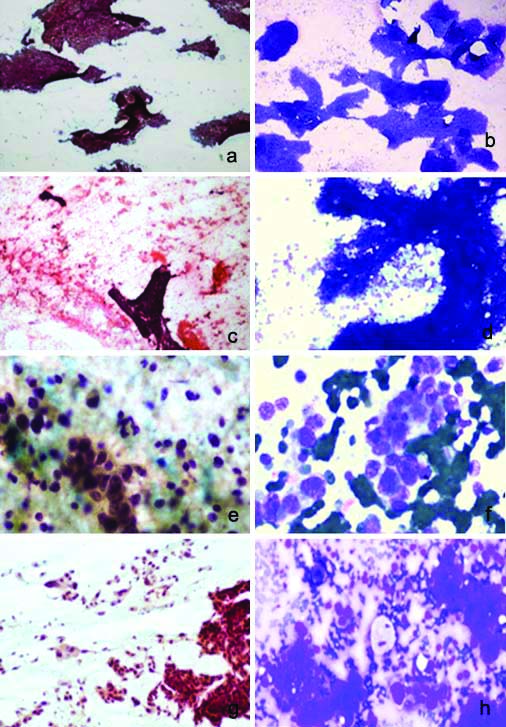
Smears showing dyscohesive clusters of atypical cells were placed in Category 3 (Atypical) [Table/Fig-3c,d]. The term atypical in breast FNAC is defined as the presence of cytological features seen predominantly in benign processes or lesions, but with some features that are uncommon in benign lesions and may be seen in malignant lesions.
Smears showing suspicious dyscohesive cells were placed in Category 4 (Suspicious of malignancy) [Table/Fig-3e,f] in a radiologically suspected case of a giant fibroadenoma. This is defined as the presence of some cytomorphological features, which are usually found in malignant lesions, but with insufficient malignant features, either in number or quality, to make a definitive diagnosis of malignancy. The type of malignancy suspected should be stated whenever possible.
Smears showing duct carcinoma were placed in Category 5 (Malignancy) [Table/Fig-3g,h]. A malignant cytological diagnosis is an unequivocal statement that the material is malignant, and the type of malignancy identified should be stated whenever possible.
Histopathology diagnosis was available in 1,069 (72.86%) cases. Among the remaining 398 cases, 320 cases did not have a histopathological diagnosis, 19 cases were lost to follow-up, 53 benign cases were on follow-up, and six cases were planned for surgery.
Respective ROM for each category was calculated [Table/Fig-4]. Sensitivity, specificity, PPV, NPV, and accuracy for each category were calculated [Table/Fig-4]. The sensitivity (86.75%), specificity (97.32%), PPV (99.19%), NPV (66.06%) was higher for the malignant category with an accuracy of 88.96% [Table/Fig-5].
Risk Of Malignancy (ROM) calculation.
| Category | Yokohama | No. of cases (n) | HPE-Benign | HPE-Malignant | ROM |
|---|
| Category 1 | Insufficient | 26 | 24 | 2 | 7.69% |
| Category 2 | Benign | 190 | 161 | 29 | 15.26% |
| Category 3 | Atypical | 78 | 27 | 51 | 65.38 % |
| Category 4 | Suspicious | 36 | 6 | 30 | 83.33% |
| Category 5 | Malignant | 739 | 6 | 733 | 99.18% |
| Total | 1069 | 224 | 845 | |
HPE: Histopathology examination
Sensitivity, specificity, PPV, NPV, and accuracy rate of histopathology diagnosis.
| Category | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|
| Category 1 | 0.24% | 90.16% | 7.69% | 20.70% | 20.39% |
| Category 2 | 2.86% | 28.12% | 12.97% | 7.17% | 8.18% |
| Category 3 | 6.04% | 87.95% | 65.38% | 19.88% | 23.20% |
| Category 4 | 3.55% | 97.32% | 83.33% | 21.10% | 23.20% |
| Category 5 | 86.75% | 97.32% | 99.19% | 66.06% | 88.96% |
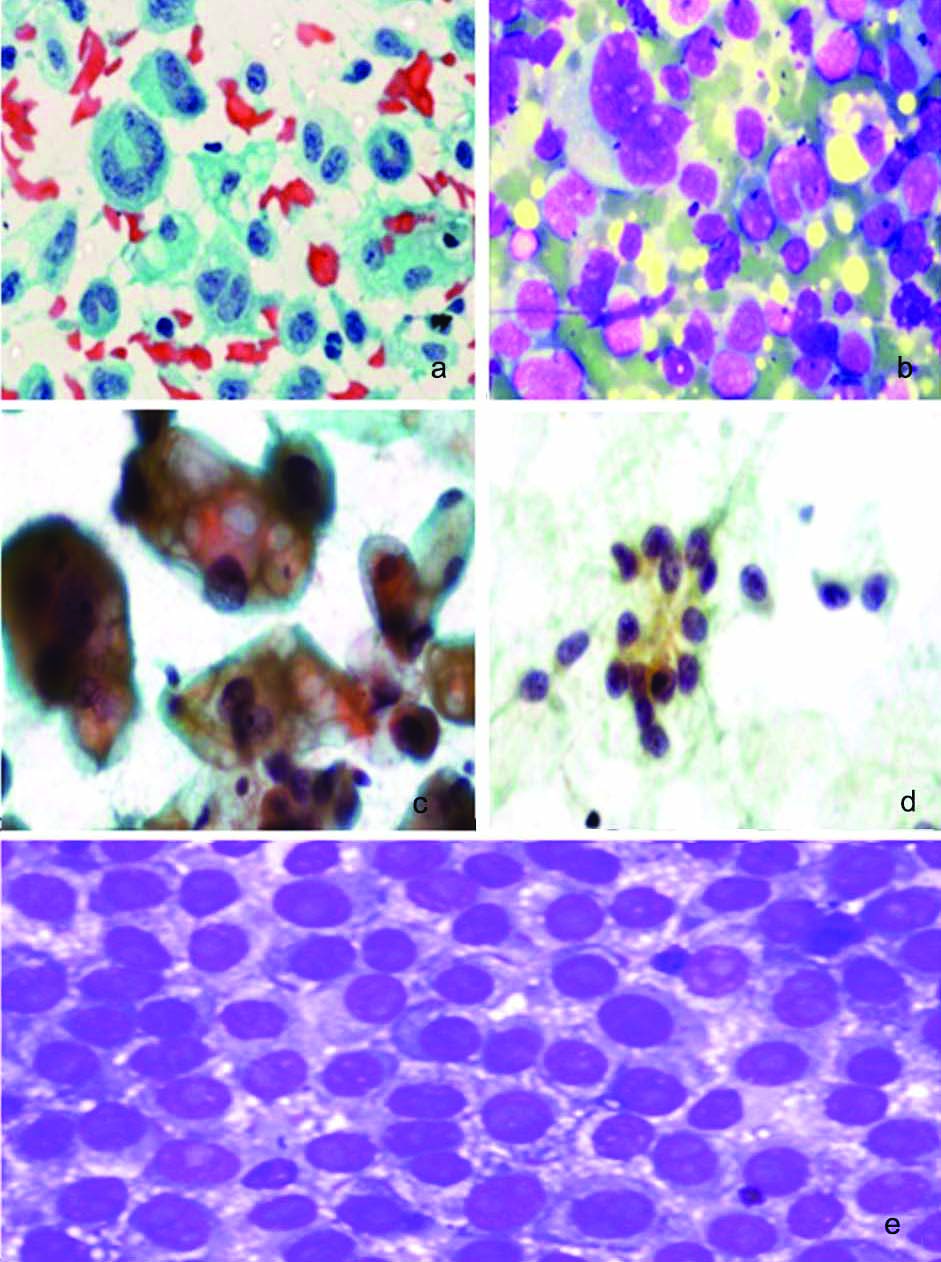
In the event of this study, author came across unusual cases of breast lesions that were diagnosed on FNAC and were compared with clinical and histopathological findings; they were 1. Anaplastic Large Cell Lymphoma (ALCL), primary thigh ulcer [Table/Fig-6a,b], 2. Metastatic adenocarcinoma, primary lung lesion [Table/Fig-6c], 3. Metastatic Ewing’s Sarcoma, primary thigh lesion [Table/Fig-6d], and 4. Metastatic melanoma, primary toe lesion [Table/Fig-6e].
a) Photomicrograph of cytology of ALCL (Pap, 40x); b) Photomicrograph of cytology of ALCL (MGG, 40x); c) Photomicrograph of cytology of metastatic adenocarcinoma lung. (Pap, 40x); d) Photomicrograph of cytology of metastatic Ewing’s Sarcoma. (Pap, 10x); e) Photomicrograph of cytology of melanoma (MGG, 40x).
Discussion
Breast cancer is the commonest malignant tumour of all female cancers, and there is increasing incidence, morbidity, and mortality globally. Although CNB is the gold standard, minimally invasive techniques like FNAC have become established for the diagnostic evaluation of palpable breast lesions. Martin and Ellis first introduced the application of FNAC for the diagnosis of palpable breast masses in 1930. Since then, it has been established as an essential tool for evaluating breast lesions [4]. A triple test consisting of clinical examination, mammography, and FNAC is considered in making a definitive assessment of breast lumps [9]. There is wide use of cytological techniques in the preoperative evaluation of breast lesions. This is because of awareness among the clinician regarding cytology techniques as a useful diagnostic tool in adjunct to clinical examination. Even FNA material can be used for ancillary techniques like immunocytochemistry and molecular testing, i.e., Progesterone Receptor (PR) and Oestrogen Receptor (ER), proliferation antigen (Ki67), and DNA pattern analysis with satisfactory results [10]. Open breast biopsies are declining, with the use of FNAC and localisation by ultrasonography. Oestrogen Receptor (ER) and PR can also be evaluated using immunocytochemistry. Alcohol-fixed direct smears can also be used for immunostaining. Molecular genetic techniques that are used in breast diagnostics on FNAC samples include Fluorescence In situ Hybridization (FISH) or chromogen ISH and Polymerase Chain Reaction (PCR). Cytology material obtained by FNAC has been shown to provide a good quality of DNA with a yield comparable to that of CNB and molecular in situ techniques performed on cytology material show an optimal concordance with histology [11]. Furthermore, FNAC specimens generally contain an even greater rate of cancer cells than core needle biopsies. The stromal component is typically more represented and might, in some cases, result in contamination and lead to bias.
FNAC of the breast and the classification system put forth by the IAC Yokohama System identifies and categorises malignant lesions and those lesions suspicious of malignancy, which was initiated with the first cytopathology group meeting in Yokohama at the 2016 International Congress of Cytology after 20 years [5]. Five categories are defined by IAC Yokohama System for reporting breast cytology, each category as a precise descriptive term, a definition, a ROM, and a management algorithm [2,5]. This System also stresses that breast FNA requires specific training and ongoing experience. With these characteristics, FNAC of the breast holds tremendous clinical value promptly and precisely triaging malignant lesions from benign lesions. This is a contemporary and global guide for the reliable diagnosis of breast lesions by FNAC. The Yokohama system, which combines clinical, radiological, and pathological findings, the triple assessment approach, followed the same criteria in present study. This approach helps assess breast lesions, which combines clinical, radiological, and pathology information to ensure accurate diagnosis and patient management [2,3,12,13]. In the present study, it was found that breast FNAC in the tertiary cancer centre is an efficacious diagnostic modality with a diagnostic accuracy of 88.96% for malignant lesions.
According to Field AS et al., the IAC Yokohama System for reporting breast FNA cytology defines five categories for reporting breast cytology. This article provides a discussion on the use of these categories [2,5]. The categories help to stratify breast lesions by their ROM and give a management algorithm for each category. The ROM was assessed and was compared with the recent studies [Table/Fig-7] [2,8,14-17]. In present study, it was observed that ROM amounted to 15.26% and 65.38% in categories 2 and 3, however, when compared with Field AS et al., we had an increased number of cases reported as atypical, probably malignant, because ours is a tertiary referral cancer centre for the state [2].
ROM of 5 categories is compared with other similar studies [2,8,14-16].
| Field AS et al., 2019 [2] | Montezuma D et al., 2019 [8] | Wai CJ et al., 2019 [14] | Wong S et al., 2019 [15] | Hoda RS and Brachtel EF, 2019 [16] | Kamatar PV et al., [17] | Present study |
|---|
| Insufficient | 2.6-4.8 | 4.8% | 2.6% | 30.3% | 0% | 7.6% |
| Benign | 1.4-2.3 | 1.4% | 1.7% | 4.7% | 4% | 15.26% |
| Atypical | 13-15.7 | 13% | 15.7% | 51.5% | 66% | 65.38% |
| Suspicious | 84.6-97.1 | 97.1% | 84.6% | 85.4, and 98.7%, | 83% | 83.33% |
| Malignant | 99.0-100 | 100% | 99.5% | 98.7%, | 99% | 99.18% |
Montezuma D et al., first-ever study to categorise breast FNAC samples based on the IAC Yokohama system categorisation [8]. Compared with the present study, ROM for category 3 was low in their study study when compared to present study [Table/Fig-7]. A retrospective study by Wong S et al., over a period of 36 months on 2,696 breast FNAC’s, where 579 cases with matched histopathology and 456 cases had Rapid Onset evaluation (ROSE), showed lower ROM for categories 2 and 3 when compared with the present study [15]. Incorporating ROSE helped them in decreasing the percentage of insufficient from 17.1% without ROSE to 4% with ROSE. Their study also had a lower ROM for categories 1 and 2.
A meta-analysis done by Hoda RS and Brachtel EF, included publications between January 1, 1997, and December 31, 2017, by reviewing literature obtained a case-cohort of 33,341 breast FNAC’s, which was drawn from 27 studies through a PubMed database [16]. They collected data for the number of total cases and each category when available and calculated ROM for each category. Their ROM for the atypical category was 51.5%, on the higher side than other mentioned studies before, however was similar to present study [2,8,14]. Their overall sensitivity and specificity were 96.3, and 98.8%, and the PPV and NPV were 98.7 and 95.3%. They concluded that the diagnostic categories of the new IAC Yokohama System each carry an implied ROM, which increases from the benign to malignant categories as observed in other studies, including the present study.
In an Indian study by Kamatar PV et al., they analysed a total of 470 cases, obtained between January 2017 and December 2018, which included 17 (4%) male patients with 453 (96%) female patients [17]. They retrospectively reviewed breast FNAC cases, and they also observed that ROM was higher in category 3, similar to the present study; however, category 1 in their study had 0 ROM.
Chauhan V et al., a study on 468 patients, wherein they had a more significant number of category 2 (Benign) cases, 342 (73.07%) and category 4, category 5 amounting to 11 (2.35%) and 85 (18.16%), when compared with present study where we had 59% (858) of category five cases [18]. They observed cyto-histopathological concordance in 98.4%, and it was 72.86% in the present study.
Sensitivity, specificity, PPV, NPV, and accuracy were compared with other studies. The sensitivity (86.75%), specificity (97.32%), PPV (99.19%), NPV (66.06%) was higher for the malignant category with an accuracy of 88.96% in the present study which was similar to Wong S et al., where sensitivity for malignant lesion was 85.3% and specificity of 100% [15].
Apart from categorising benign and malignant, IAC Yokohama also pointed out the ROM like the Bethesda system for thyroid FNAC [19].
Limitation(s)
Importantly, cases with insufficient material should undergo repeat FNAC, if the index for clinical and radiological suspicion of malignancy is high. Present institute being a referral cancer care centre, there were many malignant cases reported; this is a limitation of present study.
Conclusion(s)
In line with prior categorisation schemes, such as the Bethesda system for reporting cervical cytology and thyroid cytopathology, this newly proposed IAC Yokohama System for reporting breast cytopathology represents a simple system that allows greater diagnostic clarity and, consequently, better communication between pathologists and treating clinicians, with clear benefits for patient management. Following the IAC Yokohama system, FNA is a useful tool and requires specific training & ongoing experience. It helps in predicting the ROM. The purpose of the IAC Yokohama System for Breast FNAB Cytology is to standardise the reporting and provide uniform diagnostic categories of a common language, consistency for different practice settings, which in turn helps in management.
HPE: Histopathology examination