In 2016, it was reported 15% of the TB cases including both new and relapsed cases was due to EPTB globally [3]. EPTB accounts for more than 34% in Cambodia, 30% in Sub Saharan Africa, Australia, to 24% in the Eastern Mediterranean Region followed by 8% in the Western Pacific Region [4]. In European nations, an increase in EPTB cases was noted in 2011 to be around 22.4%. In India, it accounted for 16% of the notified cases [4].
In India, the prevalence of NTM was studied to be about 0.77% among TB suspects in New Delhi, India [5]. The prevalence among extrapulmonary suspects in tertiary care centre in Northern India was 8% [6]. Fatima S and Nm A, study conducted in Hyderabad among both pulmonary and extrapulmonary samples to find the prevalence was estimated to be 3.49% in that locality [7].
Diagnosis of EPTB always poses a challenge. SM may become negative due to its paucibacillary nature. Culture, although a gold standard method for EPTB diagnosis, it is time consuming and liquid culture like BACTEC system inturn needs separate disposal mechanisms for its radioactive waste [8]. Hence, there is an increase in use of non-radiometric method such as MGIT 960 system for the recovery of mycobacteria which eliminates the features like prolonged incubation time and the need for waste disposal. Though, molecular methods like Polmerase Chain Reaction (PCR) are available but certain disadvantages were anticipated like false positivity, inability to identify whether the bacilli is viable or dead, null information on drug susceptibility [8].
In the past, many studies were done comparing MGIT 960 with LJ media for the isolation of Mycobacterium tuberculosis complex and most of the conducted studies were from pulmonary specimens [9,10]. Hence, in the present study, authors aimed to evaluate MGIT 960 with solid LJ culture for recovery of both Mycobacterium tuberculosis complex and NTM from extrapulmonary specimens.
Materials and Methods
A prospective study was conducted by the Department of Microbiology from July 2018 to March 2020 after obtaining Institutes Ethics Committee approval (Approval No. JIP/IEC/2018/122). The current study was conducted on extrapulmonary specimens from the tuberculosis suspects sent by the treating physician. As the study was not directly connected to the patient waiver of consent was obtained. The required demographic details like age, gender of the TB suspects, type of specimen were obtained from the requisition form as decoded by the incharge of Mycobacteriology.
Inclusion and Exclusion criteria: All the extrapulmonary specimens (pleural fluid, ascitic fluid, pus aspirate, tissue biopsy, peritoneal fluid, synovial fluid, bone marrow, urine etc.,) from the tuberculosis suspects that were received in the laboratory during the study period were included. The inclusion criteria include all extrapulmonary specimens received in the Department of Microbiology for Mycobacteria culture. Insufficient samples, leaking container and samples collected if exceeds more than seven days were excluded from the study.
Initial Decontamination and Sample Processing
The extrapulmonary specimens were processed following the Revised National Tuberculosis Control Programme (RNTCP) guidelines in India. Decontamination was done by standard N-Acetyl L-Cysteine (NALC)/Sodium Hydroxide (NaOH) method [11].
Lowenstein Jensen (LJ) Media Inoculation
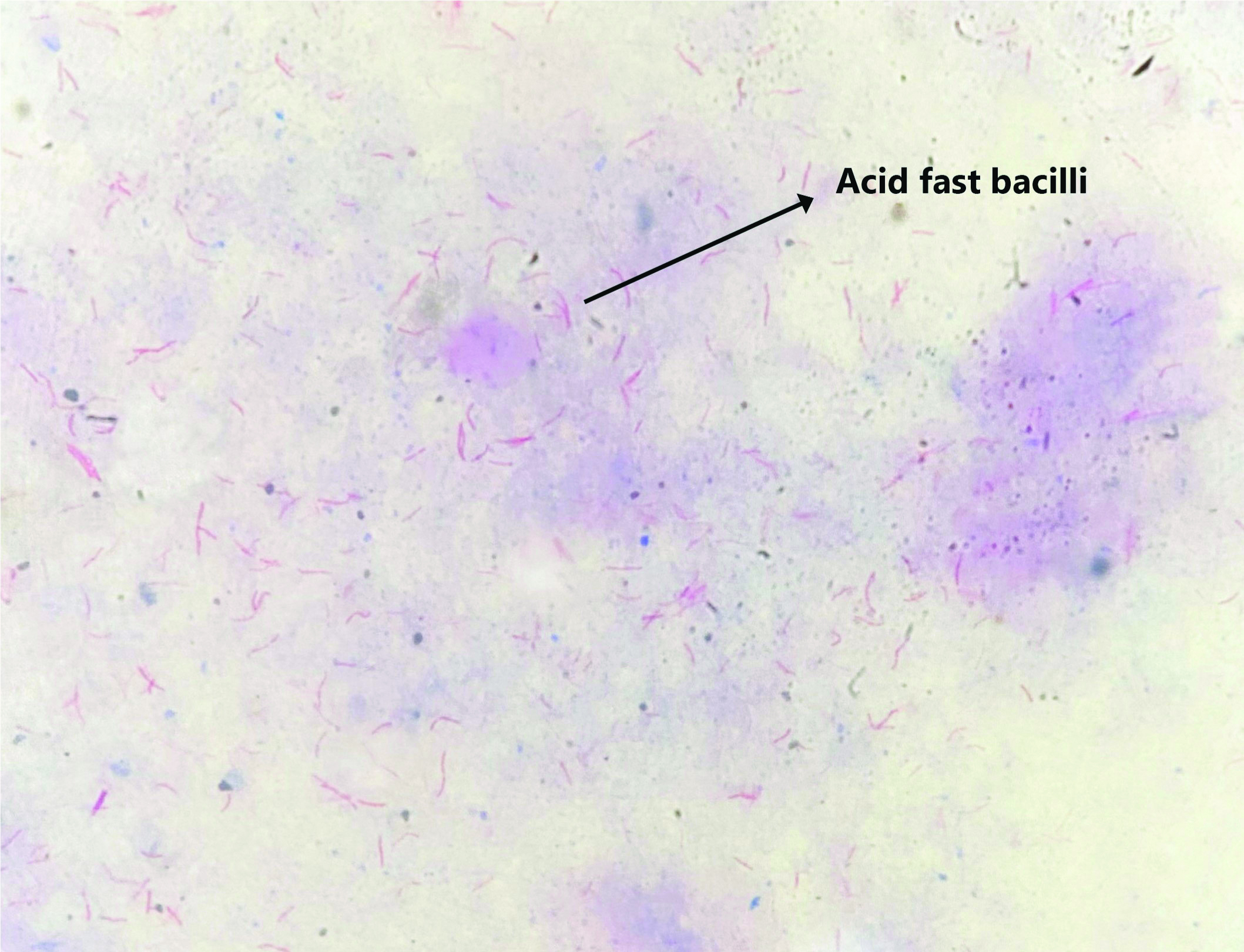
The decontaminated samples were first subjected to ZN staining and focussed under light microscope for the detection of Acid Fast Bacilli (AFB) [Table/Fig-1]. Two to three drops were inoculated into LJ medium and incubated at 37°C for eight weeks. Once a week, the LJ slants were inspected for the presence of typical mycobacterial colonies. Once the growth was seen on LJ, ZN staining for observing AFB and subculture in 5% sheep blood agar were performed to look for contamination [11].
ZN staining showing Acid Fast Bacilli (AFB) (40x).
Mycobacterial Growth Indicator Tube MGIT 960 Inoculation [
12]
Similarly, 0.5 mL of the decontaminated specimen was inoculated in MGIT 960 tubes. Inoculated MGIT 960 tubes were incubated in the MGIT 960 system (BD Bactec™ MGIT™ 960, BD Biosciences, Mumbai) for six weeks or till the tubes were flagged positive by the machine. Once the MGIT 960 was flagged positive they were also subjected to ZN staining and subculture was done on 5% sheep blood agar to look for contamination.
Positive LJ slant and MGIT 960 tubes were subjected to BD MGIT TBc Identification test (ICT) (Becton and Dickinson, New Delhi, India) for detection of MPT64 antigen. Isolates were identified as Mycobacterium tuberculosis complex if they are positive for both AFB and ICT and were identified as NTM isolates if they were positive for AFB and negative for ICT.
Statistical Analysis
Data were analysed using the SPSS® for Windows® release 21.0 (SPSS Inc., Chicago, IL, USA). Unpaired t-test was used to compare TTD for Mycobacterium tuberculosis complex and NTM by MGIT 960 and LJ. Chi-square test was performed for comparing the positivity of different specimens by MGIT 960 and LJ. The p-value <0.05 was considered to be statistically significant. The percentage agreement was calculated as (a+d)/(a+b+c+d)×100 i.e., (True Positive+True Negative)/(True Positive+False Positive+False Negative+True Negative)×100.
Results
Total 1879 extrapulmonary specimens were obtained from the clinically suspected tuberculosis patients. Of the 1879 patients, 748 (39.8%) were female patients and 1131 (60.2%) were male patients. The mean±SD age of the patients was 42.14±18.28 years. The number of patients in the age group 0-20, 21-40, 41-60, 61-80 and 81-100 were 252 (13.4%), 615 (32.7%), 730 (38.9%), 273 (14.5%) and 9 (0.5%), respectively. Out of that 129 (6.9%) grew mycobacteria by MGIT 960 and 105 (5.6%) by LJ culture. Among the mycobacteria that were grown by MGIT 960, 118 (91.5%) were identified as Mycobacterium tuberculosis complex and 11 (8.5%) as NTM. LJ culture identified 95 (90.5%) isolates as Mycobacterium tuberculosis and 10 (9.5%) as NTM [Table/Fig-2]. The sensitivity of the liquid culture MGIT 960 and solid LJ culture for Mycobacterium tuberculosis were 93.7% and 75.4%, respectively (p-value <0.0001). The sensitivity of the liquid culture MGIT 960 and solid LJ culture for NTM were 78.6% and 71.4%, respectively (p-value=1). Contamination rate associated with MGIT 960 and LJ culture was 4.6% and 4.3%, respectively and was found not to be statistically significant (p-value=0.556).
In present study, the TTD was found to be statistically significant for Mycobacterium tuberculosis detection [Table/Fig-3].
Comparison of MGIT and LJ for the recovery of mycobacteria.
| MGIT (n=1879) | LJ (n=1879) |
|---|
| Mycobacterium tuberculosis | 118 (6.3%) | 95 (5.1%) |
| NTM | 11 (0.6%) | 10 (0.5%) |
| Negative | 1664 (88.5%) | 1694 (90.1%) |
| Contaminated | 86 (4.6%) | 80 (4.3%) |
Comparison of MGIT and LJ in the estimation of Time to Detection (TTD) for MTBC and NTM.
| Organism | MGIT (Days) | LJ (Days) | p-value* |
|---|
| Mycobacterium tuberculosis | 18.45±8.06 | 33.39±10.16 | <0.0001 |
| NTM | 15.36±7.66 | 16.80±8.22 | 0.6824 |
*: Unpaired t-test
Among AFB smear positive specimens, the positivity of Mycobacterium tuberculosis by MGIT 960 and LJ were 77.3% and 72.7%, respectively (p-value=1) [Table/Fig-4]. With regard to AFB smear negative specimens, the positivity of Mycobacterium tuberculosis by MGIT 960 and LJ were 5.4% and 4.3%, respectively (p-value <0.0001). The agreement between MGIT and LJ was estimated to be 94.8% (Kappa-0.737) [Table/Fig-5].
Comparison of MGIT and LJ positivity among smear positive and smear negative specimens.
| Smear | MGIT | LJ |
|---|
| MTBC (%) | NTM (%) | NEG (%) | CON (%) | MTB (%) | NTM (%) | NEG (%) | CON (%) |
|---|
| Negative (n=1857) | 101 (5.4) | 11 (0.6) | 1661 (89.4%) | 84 (4.5%) | 79 (4.3%) | 10 (0.5%) | 1688 (90.9%) | 80 (4.3%) |
| Positive (n=22) | 17 (77.3%) | 0 | 3 (13.6%) | 2 (9.1%) | 16 (72.7%) | 0 | 6 (27.3%) | 0 |
MTBC: Mycobacterium tuberculosis complex; NTM: Non tuberculous mycobacteria; NEG: Negative; CON: Contaminant
Agreement between MGIT and LJ for detection of MTB and NTM.
| LJ | Total |
|---|
| MTB | NTM | NEG | CON |
|---|
| MGIT | MTB | 87 | 0 | 15 | 16 | 118 |
| NTM | 0 | 7 | 4 | 0 | 11 |
| NEG | 4 | 2 | 1641 | 17 | 1664 |
| CON | 4 | 1 | 34 | 47 | 86 |
| Total | 95 | 10 | 1694 | 80 | 1879 |
Kappa: 0.737; MTBC: Mycobacterium tuberculosis complex; NTM: Non tuberculous mycobacteria; NEG: negative; CON: contaminant
Detection rate for Mycobacterium tuberculosis was higher for pus in both the media and for NTM, pleural fluid demonstrated increased frequency in both the media [Table/Fig-6].
Comparative detection of mycobacterium according to the type of specimen, staining and culture medium.
| Specimen | Total No. | ZN Positive | MGIT Positive | LJ Positive | p-value* |
|---|
| NTM | MTB | NTM and MTB | NTM | MTB | NTM and MTB |
|---|
| Ascitic fluid | 180 | 2 | 0 | 9 | 9 (5.0%) | 0 | 8 | 8 (4.4%) | 1 |
| Biopsy | 142 | 4 | 0 | 12 | 12 (8.5%) | 0 | 8 | 8 (5.6%) | 0.125 |
| Blood | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Bone marrow aspirate | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Cervical swab | 4 | 1 | 0 | 1 | 1 (25.0%) | 0 | 1 | 1 (25.0%) | 1 |
| CSF | 257 | 0 | 0 | 14 | 14 (5.4%) | 0 | 13 | 13 (5.1%) | 1 |
| Endometrium | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| FNAC | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Pericardial fluid | 9 | 0 | 0 | 1 | 1 (11.1%) | 0 | 1 | 1 (11.1%) | 1 |
| Placenta | 4 | 0 | 0 | 1 | 1 (25.0%) | 0 | 1 | 1 (25.0%) | 1 |
| Pleural fluid | 680 | 3 | 5 | 27 | 32 (4.7%) | 6 | 23 | 29 (4.3%) | 0.549 |
| Pus | 264 | 8 | 2 | 34 | 36 (13.6%) | 3 | 33 | 36 (13.6%) | 1 |
| Stool | 6 | 0 | 0 | 2 | 2 (33.3%) | 0 | 2 | 2 (33.3%) | 1 |
| Synovial fluid | 30 | 0 | 0 | 1 | 1 (3.3%) | 0 | 1 | 1 (3.3%) | 1 |
| Urine | 282 | 4 | 4 | 16 | 20 (7.1%) | 1 | 4 | 5 (1.8%) | 0.001 |
| Total | 1879 | 22 | 11 | 118 | 129 (6.9%) | 10 | 95 | 105 (5.6%) | 0.001 |
*: Chi-square test; ZN: Ziehl neelsen; MGIT: Mycobacterium growth indicator tubes; LJ: Lowenstein jensen; CSF: Cerebrospinal fluid; FNAC: Fine needle aspiration cytology; NTM: Non tuberculous mycobacteria; MTB: Mycobacterium tuberculosis
Discussion
India being a highest TB burden country in the world, the prevalence of infections due to both Mycobacterium tuberculosis and NTM are both rising. Though pulmonary involvement is more common, mortality and morbidity due to extrapulmonary involvement is also increasing. As mentioned earlier due to paucibacillary nature, diagnosis by traditional conventional methods like SM, egg-based media like LJ culture is difficult due to limited sensitivity [13].
The current study showed increased isolation rate with MGIT 960 compared to LJ culture. In concordance with present study, there are similar studies that showed increased isolation with MGIT 960 compared to LJ culture. Saini D et al., study conducted on 66 extrapulmonary samples showed 46.9% isolation rate of mycobacteria by MGIT 960 and 31.8% isolation rate of mycobacteria by LJ culture [13]. Wang G et al., study on 103 extrapulmonary specimens showed the recovery rate of mycobacteria by MGIT 960 systems and LJ medium was 75.73% and 43.72%, respectively [14]. Mishra V et al., study conducted in northern India showed 94.2% were positive with MGIT-320 and 89.8% were positive LJ cultures [15]. Comparison of various studies using MGIT for detection of mycobacteria is shown in [Table/Fig-7] [13,15-17].
Literatures comparing MGIT 960 with solid media [13,15-17].
| Study (year) | Place | Sample size | Results |
|---|
| Saini D et al., 2017 [13] | Uttar Pradesh | 66 | MGIT positivity: 46.9% |
| Smaoui S et al., 2015 [16] | South Tunisia | 634 | MGIT positivity: 93.8% |
| Mishra V et al., 2016 [15] | North India (Bareilly) | 132 | MGIT positivity: 94.2% |
| Rishi S et al., 2007 [17] | Jaipur | 500 | MGIT positivity: 98.06% |
| Present study | Southern India | 1879 | MGIT positivity: 6.9% |
In this study, the TTD was found to be significantly shorter for MGIT 960 compared to LJ culture in detecting Mycobacterium tuberculosis. A study conducted in South Tunisia in extrapulmonary specimens showed the TTD was 18.5±7.6 days for MGIT 960 and for solid LJ media the time to positivity was 44.8±19.3 days [16]. Similarly a North Indian study conducted in pulmonary and extrapulmonary showed the TTD was found to be less for MGIT around 14.2 days whereas for LJ it was found to be 23.2 days [15]. The study conducted for recovery of mycobacteria in China showed the time to positivity to be 22.20±7.84 days and 42±8.84 days for MGIT 960 and LJ medium respectively [14].
The positivity for Mycobacterium tuberculosis detection by MGIT 960 and LJ in both AFB smear positive specimens and AFB smear negative specimens in the present study were comparable to study done by Pfyffer GE et al., where they showed that MGIT detected 88.6% and solid media detected 85.7% in smear-positive specimens, whereas in smear-negative specimens the recovery rate of mycobacteria MGIT and solid media was 68.2% and 59.1%, respectively [18].
Contamination rate in this study was found to be around 4.6% by MGIT 960 and 4.3% by LJ media and it was not statistically significant. There are few studies that showed decreased contamination rate with MGIT 960 compared to solid LJ media [15,16,18]. On the other hand increased contamination rate of 3.74% was reported with MGIT 960 while LJ media reported contamination of 2.80% in a study conducted among suspected EPTB patients [14]. Also, a study conducted in Hungary in a total of 377 clinical samples showed 3.7% contamination with MGIT 960 and 1.2% with LJ media [9].
In this study, 13.6% of mycobacteria were detected by both MGIT 960 and LJ culture methods in pus sample whereas in pleural fluid mycobacterial detection was found to be 4.7% with MGIT 960 and 4.3% with LJ culture. Mycobacterial detection rate in CSF sample was 5.4% and 5.1% by MGIT 960 and LJ culture respectively in present study and interestingly authors found that in urine sample, 7.1% mycobacteria were detected by MGIT 960 and 1.8% by solid LJ culture. A north Indian study reported 77.7% from pus, 69.5% of Mycobacterium tuberculosis complex from pleural fluid, 18.1% from CSF sample conducted among 66 extrapulmonary specimens [8]. Chan DS et al., study conducted in 1393 urine specimens showed 4.1% of mycobacteria to be isolated by MGIT 960 system [10]. Hillemann D et al., study among 9558 extrapulmonary specimens documented 2.7% of mycobacteria from cerebrospinal fluid and 2.6% from the urine specimens [19]. In this study, it was observed that for urine samples, MGIT 960 showed increased isolation rate compared to solid media. But the isolation rate of other extrapulmonary specimen was similar by both culture media.
Limitation(s)
The main limitation of the study was that the clinical information of the patients was not collected, as this study was based on the samples routinely sent to the laboratory for culture and the minimal clinical details such as age and gender were entered in the requisition form.
Conclusion(s)
Present study found an increased recovery rate with MGIT 960 compared to LJ culture. The TTD for Mycobacterium tuberculosis complex was found to be statistically significant and the contamination rate associated with both culture methods was similar. In the future, in case of tertiary care settings MGIT 960 alone can be used for culture of extrapulmonary specimens for the fast recovery of mycobacteria.
*: Unpaired t-test
MTBC: Mycobacterium tuberculosis complex; NTM: Non tuberculous mycobacteria; NEG: Negative; CON: Contaminant
Kappa: 0.737; MTBC: Mycobacterium tuberculosis complex; NTM: Non tuberculous mycobacteria; NEG: negative; CON: contaminant
*: Chi-square test; ZN: Ziehl neelsen; MGIT: Mycobacterium growth indicator tubes; LJ: Lowenstein jensen; CSF: Cerebrospinal fluid; FNAC: Fine needle aspiration cytology; NTM: Non tuberculous mycobacteria; MTB: Mycobacterium tuberculosis