The desire for whiter teeth among the general population has reached an all-time high with many individuals seeking professional help for non-invasive bleaching techniques. Despite various potential side-effects of peroxide, it has remained the active ingredient in tooth whitening agents in the form of HP and carbamide peroxide [1]. There is evidence to show that 35% HP results in shallow erosions, sensitivity, structural alterations in enamel structure and compromised bonding of resin composite [2,3].
Attempts to minimise these adverse effects by incorporating remineralising agents, calcium and fluoride substitutes, the results have met with limited success [4]. Furthermore, compromised bonding of adhesive restorations to bleached teeth is an important clinical concern. The use of antioxidants and proanthocyanidins appear to completely neutralise the deleterious effects of bleaching and increases the micro-shear bond strength significantly [5-8]. However, it is important to note that these adjunct treatments were unable to reverse the structural damage of enamel surface caused by the bleaching agent [9].
Recently there has been an upsurge in usage of natural substances for treatment of various diseases as they minimise or prevent the side effects [10,11]. Similarly, this has led to a constant search for replacing the existing chemicals in cosmetic dentistry used for various purposes. This change would be welcomed in dentistry if there is an alternative for HP due to its various adverse effects on the enamel surface.
Strawberry (fragaria spp.) a herbaceous perennial fruit plant belonging to the family “Rosaceae” is grown in many countries. Apart from its various benefits exerted on the well-being of an individual, it is been noted for its natural tooth whitening potential due to its low pH and presence of various constituents namely citric and malic acid as stated by Chacko K et al., in (2018) and Aub M (2010) [10,12].
However, it remains unknown if SE, alone or in combination with HP can achieve the same bleaching effect as HP alone, without the disadvantages of HP such as surface erosion and compromised bonding of resins. Hence, this study was designed to comparatively investigate the effect of SE, with and without HP on the bleaching effectiveness, surface characteristics and micro-shear bond strength of resin composite to bovine enamel and to compare these results with HP bleached enamel, treated with proanthocyanidin to determine its effects on the bleached enamel. The null hypotheses were that: (1) SE does not significantly improve bleaching, compared to HP alone; and (2) SE does not improve the micro-shear bond strength of resin composite to enamel.
Materials and Methods
This invitro study was conducted in the Department of Conservative Dentistry and Endodontics, SRM Dental College and Hospitals, Chennai, Tamil Nadu, India. The study was started in January 2013 and was conducted over a time period of nine months. Study protocol was approved by the Institutional Review Board and Ethical Committee of the University. (SRMU/M&HS/SRMDC/2013/M.D.S/PG/304). Bleaching efficacy and micro-shear bond strength of SE was evaluated. Using IBM SPSS Statistics, version 22 with power of 80% and alpha error 5%, the sample size was calculated.
Preparation of Experimental Solutions
The fresh pulpy strawberry was procured from the market (Chennai). A 200 gm of strawberry fruits were cleaned, cut into cubes and blended with 15 mL of distilled water in a blender to obtain about 100 mL of strawberry concentrate. This concentrate was filtered and then transferred into a cooling centrifuge to be processed at 2000 rpm for about 20 minutes at a temperature of 40C. The clear liquid thus collected (2% SE) was stored at 4oC till further use. Similarly, 5 gm of grape seed extract in the form of powder was collected from capsule (Puritans Pride Inc, Oakdale, NY, USA) and dissolved in 100 mL of distilled water to obtain 5% proanthocyanidin solution (P) [Table/Fig-1,2,3 and 4] [13].

Strawberry and Strawberry concentrate.
Centrifuge and Strawberry concentrate in centrifuge machine.

Proanthocyanidin powder and Solution.
Specimen Collection
Bovine teeth were used in this study due to similarities with that of human enamel (Nakamichi I et al., 1983) and the ease of obtaining these specimens [14]. The large surface area provided by the bovine teeth enabled us to obtain 4 sections from a single tooth for uniformity of the enamel surface for the groupings. Single rooted, caries free, bovine maxillary central incisor teeth (n=30) were taken (from the butcher’s shop), teeth with fractures, cracks and stains were excluded. The experiments were performed in accordance with the appropriate guidelines [13]. The collected teeth were stored in 0.2% thymol (Aston Fine Chem, Chennai) and kept hydrated until use.
Staining of Teeth and Bleaching Protocol
The teeth were sectioned at the cemento-enamel junction so as to obtain the crown portion and were stored in saline until it was subjected to further experimental procedures. A 15 teeth served as negative control (group 1-N) and did not receive any bleaching procedure nor staining.
The remaining 15 experimental specimens (group-2) were stained artificially based on a previously established protocol as described by Sulieman M et al. A 2 g tea bag (Ranfer Tea, Colombo, Sri Lanka) was suspended in boiling de-ionised water (100 mL) for 5 minutes and then it was cooled down to room temperature. The clear solution [3] followed by straining the solution was used for staining the teeth. Clear tea solution was obtained by straining the solution. The specimens were then immersed in the tea solution for 24 hours, after which they were removed and subjected to reflectance spectrometer (X-Rite Gretag Macbeth, Berlin, Germany) to record the baseline colour variables (pre-bleach) (L*, a*, b*-∆E values). The crowns were then sectioned longitudinally and horizontally to obtain four equal parts (60 specimens) [Table/Fig-5]; each part being randomly allocated to one sub-group (a,b,c,d; n=15 each), depending on the bleaching protocol as stated below [13]. The specimens were mounted on self-curing acrylic resin blocks, exposing only the labial surface.
Group 1 (n=15)
Negative control (N)
Group 2 (n=60)
Group 2a (n=15): 35% HP (Thermo fisher scientific India Pvt., Ltd., Mumbai, India)
Group 2b (n=15): 2% SE
Group 2c (n=15): HP+SE (1:1) (HPS)
Group 2d (n=15): HP followed by 5% Proanthocyanidin solution (P) (HPP)
The labial surface of the specimens of group 2 was exposed to the respective bleaching agent for 5 minutes using cotton tip applicator replenishing every minute. The teeth were then rinsed and stored in artificial saliva at room temperature for 24 hours [13]. All the specimens were further subjected to reflectance spectrometer to obtain the post-bleach colour variables L*, a*, b* according to the CIE (Commission Internationale de l’eclairage) L*a*b colour system. The colour change (ΔE) was calculated using the formula [13]:
ΔE={(ΔL*) 2+(Δa*) 2+(Δb*) 2}½, Where L*, a*, b* represents value of colour using hue, value, and chroma
To evaluate the whiteness after bleaching, the colour changes before and after bleaching was taken as an index [13]. All the procedures were performed at room temperature by the same operator under aseptic conditions.
Scanning Electron Microscopic Analysis
To analyse the effect of bleaching solutions on the surface morphology of enamel, 2 of 15 specimens of all groups were subjected to Scanning Electron Microscopic analysis (SEM JEOL model, JSE-5610 LV). The specimens were vacuum desiccated first in alcohol (Alpha Chemika Mumbai, India) and subsequently in acetone (Prasol Chemicals Private Limited, Mumbai) and finally sputter-coated with gold (Rare Earth, Mumbai, India). Micrographs were exposed at a magnification of 1000X. The complete labial surface area was scanned and random areas were selected to be scanned for Scanning Electron Microscope (SEM) images.
Micro-Shear Bond Evaluation
The remaining specimens from each group (n=13) were etched with 37% phosphoric acid for 15 seconds, rinsed with water for 20 seconds, and bonded with self-etch adhesive (Adper Easy One, 3M ESPE, Germany). This was followed by composite build-up of 5 mm height and 3 mm diameter using Teflon mold (Filtek Z350XT, 3M ESPE, Dental Products, USA). The enamel composite blocks were sectioned to obtain 1×1 mm sticks [Table/Fig-5] using a low speed diamond disc under water coolant. The enamel-composite interface of the specimen was subjected to shear load using Instron Universal Testing Machine (LR 100K, Lloyd Instruments, Largo, FL, USA) at a cross head speed of 1mm/min. The obtained values were recorded in MPa.
Statistical Analysis
Data were analysed with SPSS software. (IBM SPSS Statistics, version 22). Kruskal-Wallis One-way ANOVA and Post-hoc Tukey’s test were used to test the statistical significance. The p-value was set at 5%.
Results
The null hypothesis of the study was rejected. The results of colour change and micro-shear bond strength values of this in-vitro study are given in [Table/Fig-6,7,8,9,10 and 11], respectively. SEM image is represented in [Table/Fig-12a-e].
Descriptive statistics comparing the mean and standard deviation of pre-bleach ∆E values for all the group 2 specimens.
| Groups | N | Mean | Std. Dev | Std.Error | 95% Confidence interval for mean | Minimum | Maximum |
|---|
| Lower bound | Upper bound |
|---|
| 2a (HP) | 15 | 11.59 | 0.33 | 0.08 | 11.40 | 11.77 | 11.10 | 12.12 |
| 2b (SE) | 15 | 11.55 | 0.43 | 0.11 | 11.31 | 11.79 | 11.00 | 12.20 |
| 2c (HPS) | 15 | 11.59 | 0.22 | 0.05 | 11.46 | 11.71 | 11.20 | 12.10 |
| 2d (HPP) | 15 | 11.67 | 0.29 | 0.07 | 11.51 | 11.84 | 11.32 | 12.21 |
HP: Hydrogen peroxide; SE: Strawberry extract; HPS: Hydrogen peroxide and strawberry; HPP: Hydrogen peroxide and proanthocyanidin solution
Descriptive statistics comparing the mean and standard deviation of post-bleach ∆E values for group 2 specimens.
| Groups | N | Mean | Std. Dev | Std.Error | 95% Confidence interval for mean | Minimum | Maximum |
|---|
| Lower bound | Upper bound |
|---|
| 2a (HP) | 15 | 19.06 | 0.52 | 0.13 | 18.76 | 19.35 | 18.02 | 20.10 |
| 2b (SE) | 15 | 18.73 | 0.38 | 0.09 | 18.52 | 18.95 | 18.04 | 19.56 |
| 2c (HPS) | 15 | 21.13 | 2.29 | 0.59 | 19.86 | 22.40 | 19.09 | 29.04 |
| 2d (HPP) | 15 | 17.23 | 0.46 | 0.12 | 16.97 | 17.49 | 16.48 | 17.94 |
Comparison of the mean and standard deviation of pre and post-bleach ∆E values for group2 specimens. Post-Hoc test was used to identify the significant groups. p-value (<0.05)
| Groups | Pre-bleach (ΔE values) | Post-bleach (ΔE values) | Difference (ΔE values) | p-value |
|---|
| Mean±SD |
|---|
| 2a (HP) | 11.59±0.33 | 19.06±0.52 | 7.47±0.19 | <0.05 |
| 2b (SE) | 11.55±0.43 | 18.73±0.38 | 7.18±0.05 |
| 2c (HPS) | 11.59±0.22 | 21.13±2.20 | 9.54±1.98 |
| 2d (HPP) | 11.67±0.29 | 17.23±0.46 | 5.56±0.17 |
Multiple comparison between post bleach mean ∆E values for group 2 specimens.
| Dependent variable | Groups | Mean difference | p-value |
|---|
| Delta E(ΔE)p | 2a (HP) | 2b (SE) | -2.19 | 0.100 |
| 2c (HPS) | -1.30 | 0.050* |
| 2d (HPP) | -2.17 | 0.001* |
| 2b (SE) | 2c (HPS) | 0.09 | 0.342 |
| 2d (HPP) | 0.02 | 1.000 |
| 2c (HPS) | 2d (HPP) | -0.08 | 0.029* |
*p-value (<0.05) are significant
Comparison of mean and standard deviation for micro-shear bond strength values in MPa for all the groups.
| Groups | N | Mean | Std. Dev | Std. Error | 95% Confidence interval for mean | Minimum | Maximum |
|---|
| Lower bound | Upper bound |
|---|
| I (N) | 13 | 36.2038 | 0.81993 | 0.22741 | 35.7084 | 36.6993 | 34.71 | 37.51 |
| 2a (HP) | 13 | 27.3854 | 1.07273 | 0.29752 | 26.7371 | 28.0336 | 25.32 | 29.10 |
| 2b (SE) | 13 | 29.4877 | 1.24296 | 0.34474 | 28.7366 | 30.2388 | 27.30 | 31.51 |
| 2c (HPS) | 13 | 28.6138 | 0.97182 | 0.26953 | 28.0266 | 29.2011 | 27.20 | 30.25 |
| 2d (HPP) | 13 | 29.5338 | 1.59527 | 0.44245 | 28.5698 | 30.4979 | 27.32 | 33.50 |
Multiple comparisons of mean micro-shear bond strength values among the experimental groups (MPa). One-way ANOVA was used to calculate the p-value.
| (I) Groups | (J) Groups | Mean difference (I-J) | Std. Error | Sig. | 95% Confidence interval |
|---|
| Lower bound | Upper bound |
|---|
| 1(N) | 2a (HP) | 8.81846* | 0.45934 | <0.001*** | 7.5266 | 10.1103 |
| 2b (SE) | 6.71615* | 0.45934 | <0.001*** | 5.4243 | 8.0080 |
| 2c (HPS) | 7.59000* | 0.45934 | <0.001*** | 6.2981 | 8.8819 |
| 2d (HPP) | 6.67000* | 0.45934 | <0.001*** | 5.3781 | 7.9619 |
| 2a (HP) | 1 (N) | -8.81846* | 0.45934 | <0.001*** | -10.1103 | -7.5266 |
| 2b (SE) | -2.10231* | 0.45934 | <0.001*** | -3.3942 | -0.8104 |
| 2c (HPS) | -1.22846 | 0.45934 | 0.070 | -2.5203 | 0.0634 |
| 2d (HPP) | -2.14846* | 0.45934 | <0.001*** | -3.4403 | -0.8566 |
| 2b (SE) | 1(N) | -6.71615* | 0.45934 | <0.001*** | -8.0080 | -5.4243 |
| 2a(HP) | 2.10231* | 0.45934 | <0.001*** | .8104 | 3.3942 |
| 2c(HPS) | 0.87385 | 0.45934 | 0.327 | -0.4180 | 2.1657 |
| 2d(HPP) | -0.04615 | 0.45934 | 1.000 | -1.3380 | 1.2457 |
| 2c (HPS) | 1 (N) | -7.59000* | 0.45934 | <0.001*** | -8.8819 | -6.2981 |
| 2a (HP) | 1.22846 | 0.45934 | 0.070 | -0.0634 | 2.5203 |
| 2b (SE) | -0.87385 | 0.45934 | 0.327 | -2.1657 | 0.4180 |
| 2d (HPP) | -0.92000 | 0.45934 | 0.277 | -2.2119 | 0.3719 |
| 2d (HPP | 1 (N) | -6.67000* | 0.45934 | <0.001*** | -7.9619 | -5.3781 |
| 2a (HP) | 2.14846* | 0.45934 | <0.001*** | 0.8566 | 3.4403 |
| 2b (SE) | 0.04615 | 0.45934 | 1.000 | -1.2457 | 1.3380 |
| 2c (HPS) | 0.92000 | 0.45934 | 0.277 | -0.3719 | 2.2119 |
Post-Hoc Tukey HSD was used to identify the significant groups. p-value (<0.05). *** denotes highly significant; HPS: Hydrogen peroxide and strawberry; HPP: Hydrogen peroxide and proanthocyanidin solution
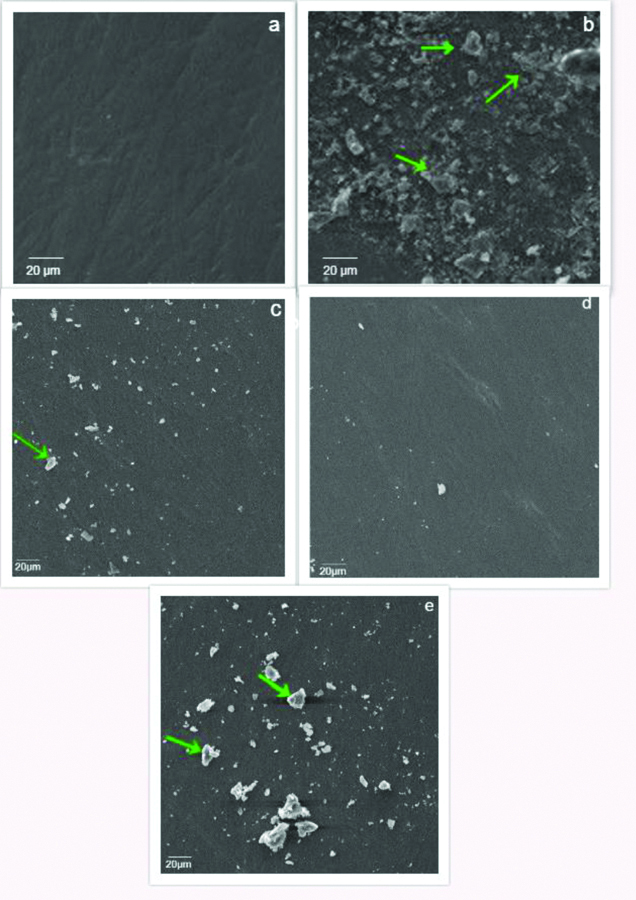
a- Group 1(N) No visible surface changes observed; b- Group 2a (HP) More roughened enamel surface with the presence of few dissolved particles (green arrow); c- Group 2b (SE) Lesser number of irregularities on the enamel surface with few particles (green arrow); d- Group 2c (HPS) Minimal morphological surface irregularities with absence of cracks; e- Group 2d (HPP) Lesser surface irregularities observed with presence of debris (green arrow).
Higher the ∆E values, better is the bleaching efficacy, i.e., more whiter is the tooth. In group 2, post-bleach ∆E values of all the specimens were significantly higher than all the respective ∆E pre-bleach specimens. (p<0.05) [Table/Fig-8].
Among the post-bleach specimens, highest and the lowest ∆E values were observed in-group 2c (HPS); (21.13±2.29) and group 2d (HPP); (17.23±0.46) respectively (p<0.05) [Table/Fig-7].
The SEM micrographs of HP showed more roughened enamel surface with presence of cracks [Table/Fig-12b]. Lesser number of irregularities were observed in group 2b (SE) and 2d (HPP) [Table/Fig-12c,e]. Minimal morphological surface irregularities with absence of cracks were observed in group 2c (HPS) [Table/Fig-12d] which was comparable to the unbleached specimens of group 1 [Table/Fig-12a].
The control (group 1) showed significantly higher micro-shear bond strength (36.20±0.81MPa) compared to the experimental groups 2a (27.38±1.07MPa); 2b (29.48±1.24MPa); 2c (28.61±0.97MPa) and 2d (29.53±1.59MPa) (p<0.05) [Table/Fig-10]. Among the groups, the micro-shear bond strength of group 2a (27.38±1.07 MPa) was significantly lesser than groups 1, 2b and 2d but not significantly different from group 2c (p>0.05) [Table/Fig-11]. Both the antioxidants (strawberry and proanthocyanidin) showed significantly higher micro-shear bond strength compared to 35% H2O2 (p<0.05).
Similarly minimal morphological surface irregularities were observed in HPS. Significantly higher micro-shear bond strength was observed in all the antioxidant group SE, HPS, HPP compared to HP.
Discussion
Hydrogen Peroxide (HP) is the most commonly used dental bleaching agent. Ionic dissociation of HP releases free radicals namely nascent oxygen, per-hydroxyl, hydroxyl radical and superoxide anions [15,16]. These highly reactive radicals disrupt the electron conjugation by breaking down the highly conjugated organic molecules involving carbon, nitrogen and oxygen atoms, into smaller, less pigmented ones, thus changing the absorption energy of the molecule [17]. The consequent shift of the visible absorption spectrum of compound from a longer to a shorter wavelength (a colourless compound) forms the basis of whitening/bleaching action on the substrate. However, the permeability of enamel to small ions (Atkinson HF 1947), results in the formation of peroxide apatite [18]. These residual peroxides interfere with resin polymerisation and adversely affect the micro-shear bond strength of the resin composite when placed immediately [19-21]. Owing to these problems, substances with minimal deleterious effect on the tooth surface are a welcome change in dentistry.
Strawberry, a widely grown hybrid species of genus Fragaria contains phytochemicals such as ellagitannin, agrimoniin, fisetin, and other polyphenols including flavonoids, such as anthocyanins and flavanols; phenolic acids such as hydroxybenzoic acid and hydroxycinnamic acid [12-22]. Several of these compounds are potent antioxidants. Citric acid (885), maleic acid and ellagic acid comprise the acid content [10,12]. In this study, it was demonstrated that SE was able to whiten enamel without altering the surface characteristics, and more importantly, without compromising the bonding of adhesive restorations.
With regards to the bleaching effectiveness, pre-bleaching values of all the groups were not significant, implying equal distribution of specimens within the groups. Post-bleaching delta E values of all the groups were statistically significantly higher compared to the pre-bleach values (p<0.05). Higher the delta E values, better is the stain removal hence more whiter is the surface of the teeth. Thus, post-bleach specimens were more whiter compared to pre-bleach specimens. This suggests that all treatments bleached effectively the tea-stained enamel (more whiter). All treatment groups (post-bleach), showed statistically significant difference in the ∆E values (p<0.05). When comparing the bleaching agents alone, the teeth bleached with SE were as white as compared to HP, i.e., it was not statistically significant (p>0.05), implying that SE had equal bleaching efficacy as the standard HP.
Song and palmer in 2008 have reported that many fruits namely strawberries, lemon, apples, pears etc., contain H2O2 during their metabolism process [23]. Apart from HP, strawberry also has ellagic acid (C14H6O8) possessing potential OH clusters, these clusters is claimed to act as a powerful oxidiser during tooth whitening process. The OH and H radicals gets released from the ellagic acid and reacts with the organic molecules disrupting the electron conjugation, thereby changing the energy absorption by forming smaller organic molecules with lighter colour [24]. OH clusters in ellagic acid compared to carboxylic group (COOH) clusters (present in other acids) have larger electronegativity and breaks easily to react with the organic molecules of the tooth enamel. The more the ellagic acid in a fruit the more effective is the bleaching process. Ellagic acid in strawberries range to around 0.43-4.64 mg/g dry weight compared to only around 0.13 mg/g in other fruits [24]. These can be substantiated with the results obtained in this study. Hence, strawberry seems to be a good alternative for tooth bleaching. Based on these results, our first null hypothesis was rejected.
HP (35%) appears to have a concentration-dependent bleaching effectiveness, which is attributed to higher content of the low molecular weight oxygen free radicals (34.014 g/mol) [23,25]. The similar efficacy of SE as compared to HP may be attributed to its pH (3.27-3.86) and the presence of the acidic components namely ascorbic acid [12]. Ascorbic acid in strawberry may increase the surface energy by removing the surface debris, thereby allowing the citric and maleic acid to penetrate into the tooth surface to remove the surface stains. This may also explain the synergistic colour change in the combination group (HPS). Both peroxide and the acids could have caused disruption of more number of complex stain molecules into smaller simpler molecules making it colourless [1]. Specimens treated with HP followed by proanthocyanidin (HPP) showed the least improvement in ∆E values and the differences were statistically significant compared to HPS (p<0.05). Indeed, this was expected as proanthocyanidin alone have not been reported to have any bleaching activity. It may also be so that the free radical scavenging action of proanthocyanidin, may have hindered the bleaching efficacy of H2O2 [24,26].
This study used SEM to analyse the surface characteristics of bleached enamel. HP has a pH ranging between 4.0-7.5 based on their peroxide concentration. The greater the peroxide concentration, the more acidic is the solution. Enamel demineralisation begins at pH less than 5.2, and thus, high concentrations of HP could have caused enamel erosion [25-28]. SEM images of the groups treated with HP or HPP showed significant surface roughness [Table/Fig-12b,e]. Interestingly, specimens where HPS was used did not show significant surface roughness [Table/Fig-12d].
Natural antioxidants occur in all parts of plants. The phytochemicals namely phenolics, anthocyanins and other flavonoids that are present in plant tissues are responsible for the antioxidant capacity [21,27,29]. Wang SY and Lin HS, has stated that extracts of berry fruits had good source of natural antioxidants with remarkable high scavenging activity towards superoxide radicals generated chemically [22]. Moreover among the berries, the highest total antioxidant capacity (Oxygen Radical Absorbance Capacity-ORAC) has been observed for strawberries [29,30]. While the antioxidants in the SE may have counteracted the adverse effects of free radicals on the enamel matrix, these results imply that such effects are achieved without any reduction in bleaching efficiency.
Specimens treated with HP alone showed the lowest micro-shear bond strength of resin composite. This may be attributed to the oxidation of organic and inorganic components, and dissolution of enamel mineral content following the application of higher concentration of HP. These findings are in accordance with the results of previous studies by Sulieman M et al., Seghi RR and Denry I and Pinto CF et al., [3,17,26,28]. While one may expect that this group, with its increased surface roughness, may also result in high micro-shear bond strength values, the presence of residual oxygen on the enamel surface, prevents complete polymerisation of the resin, resulting in poor bonding [22,31].
Specimens treated with SE, HPS and HPP showed increased micro-shear bond strength values compared to those treated with HP alone. It can be inferred that, the restoration can be done immediately following the bleaching procedure. Hence, two weeks of waiting period can be avoided, to nullify the presence of oxygen radicals which otherwise could result in incomplete polymerisation. Based on the results of this study, the second null hypothesis was also rejected. However, these micro-shear bond strength values were still significantly less than the untreated controls, indicating that once bleached, any attempts at reversal may not increase micro-shear bond strength values to levels comparable to untreated enamel. The efficacy of SE and proanthocyanidin in reversing the compromised micro-shear bond strength may be attributed to flavonoids, which function as scavengers of free radicals by rapid donation of hydrogen atoms to radicals [30-34].
Limitation(s)
As the present research represents the first report comparing the bleaching efficacy of SE with HP and bonding of resin composite following bleaching, the actual depth of resin penetration by means of Confocal Laser Scanning Microscopy (CLSM), the possible chemical mechanism and its long term efficacy remains a major limitation of this study. Also, other limitation of this study is that, the SEs bleaching efficacy was explored only on tea stained enamel surface, whereas it would have been interesting to explore its activity in non-vital discoloured teeth, fluorosis, which will be, indeed, a possible aim of our future research.
Conclusion(s)
This study highlights that enamel bleaching with SE, effectively bleaches the surface, without significant adverse effects on the enamel surface and allows for immediate bonding procedures without compromising the micro-shear bond strength of the resin.
HP: Hydrogen peroxide; SE: Strawberry extract; HPS: Hydrogen peroxide and strawberry; HPP: Hydrogen peroxide and proanthocyanidin solution
*p-value (<0.05) are significant
Post-Hoc Tukey HSD was used to identify the significant groups. p-value (<0.05). *** denotes highly significant; HPS: Hydrogen peroxide and strawberry; HPP: Hydrogen peroxide and proanthocyanidin solution