Regional blocks have secured a pivotel role in modern anaesthetic practice with their ability to achieve ideal operative conditions without much of systemic side effects. They offer an excellent alternative for patients who are haemodynamically compromised or too ill to tolerate general anaesthesia [1]. The effectiveness in terms of success rates, margin of safety and efficient post-operative analgesia are the main reasons which made supraclavicular brachial plexus blockade, a technique of choice for most of the upperlimb surgeries performed on a day to day basis [2]. The first supraclavicular brachial plexus block was performed by Kulenkampff D in 1912 [3].
Levobupivacaine is a newer local anaesthetic with less cardiotoxic effects and a similar duration of action compared to bupivacaine [4]. This favourable clinical profile has prompted many clinicians to choose levobupivacaine over bupivacaine for all types of neural blockade. Dexmedetomidine, a pharmacologically active dextroisomer of medetomidine, is a selective α2-adrenoceptor agonist employed for use as an adjuvant during locoregional anaesthesia. It also has a potent opioid sparing effect, reducing the opioid requirements significantly both during intraoperative and postoperative periods [5,6]. A few prevailing literature has demonstrated that adding dexmedetomidine to local anaesthetics significantly improves the quality of central neuraxial blockades like subarachnoid, epidural, and caudal anaesthesia, but there is paucity in literature for its usage in peripheral nerve blockades [7-10].
Hence, this study aimed to evaluate the clinical effects of addition of dexmedetomidine to levobupivacaine and levobupivacaine alone in patients undergoing elective upperlimb surgeries under supraclavicular brachial plexus blockade. The primary outcomes assessed were the time of onset and duration of sensory and motor blockade along with duration of post-operative analgesia; and secondary outcomes were haemodyamic parameters namely Heart Rate (HR), Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP).
Materials and Methods
This randomised controlled study was conducted in Department of Anaesthesiology, JN Medical College, KLE Academy of Higher Education and Research, Belagavi, Karnataka, India between the period of January 2014 to December 2014. The study was approved by the Institutional Ethics Committee (reference letter no.- IEC-MDC/DOME/10), prior to the commencement and written informed consent from the patients was obtained.
Inclusion criteria: Fifty ASA I and II patients between the age group 18 and 60 years undergoing elective forearm and hand surgeries were included.
Exclusion criteria: Patients with infection at the site of injection, clinically significant coagulopathy, pre-existing neuromuscular disorders, severe cardiovascular or pulmonary diseases, renal or hepatic disorders, pregnancy and lactation and those taking opioids or chronic analgesic therapy for any other illnesses were excluded from the study.
The formula used for sample size calculation is;
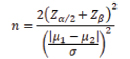
Where, μ1 is mean of the first group, μ2 is mean of the second group, σ is the common error variance, Zα/2 value is 1.96 for 95% confidence level and Zβ value is 1.2816 for 90% power. By considering onset of motor block in group LD 50 and group LD 100 as 19.75±6.3 minutes and 14.3±4.2 minutes respectively from previous study, at 5% level of significance, and 90% power, the sample size was obtained to be 22 subjects per each group [11]. Total sample size required was 22×2=44 subjects. As sample size increases, accuracy of result increases. So, 50 samples were considered in this study (25 in each group).
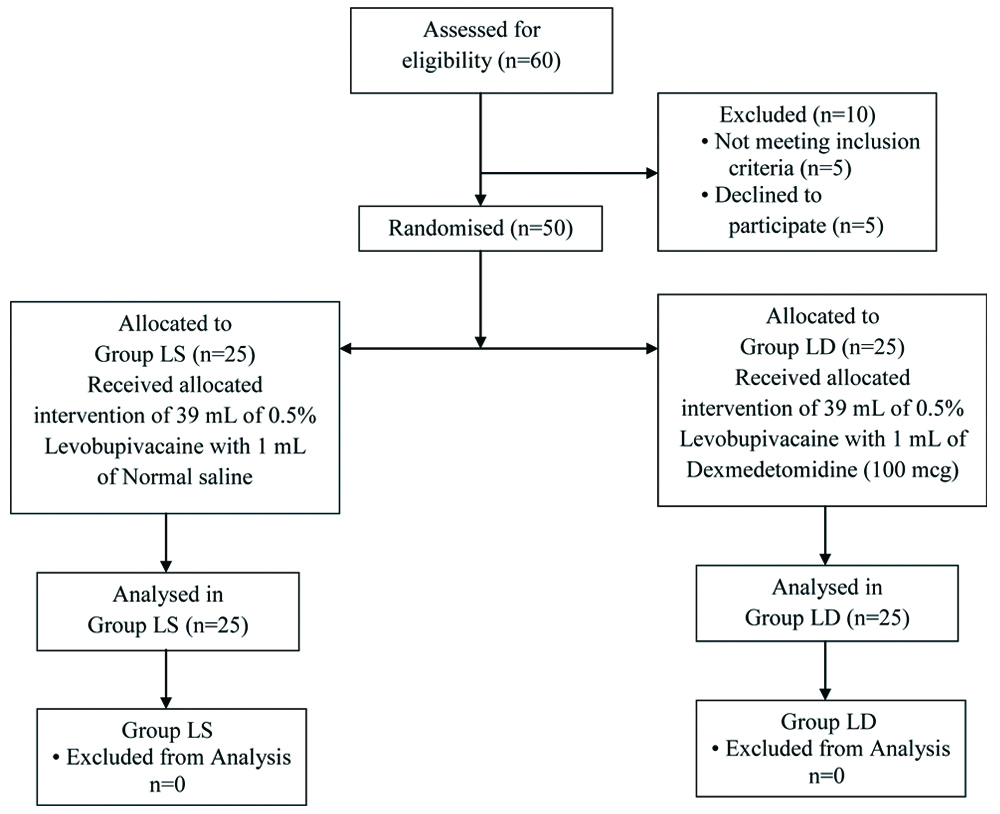
Randomisation: Patients were allocated in a randomised manner by computer generated randomisation chart into two groups of 25 each as group LS to receive levobupivacaine with normal saline and group LD to receive levobupivacaine with dexmedetomidine. A consort flow diagram of patients and their progress through the various phases of this randomised trial is outlined in [Table/Fig-1].
Intervention: Group LS received 39 mL of 0.5% levobupivacaine + 1 mL normal saline and group LD received 39 mL of 0.5% levobupivacaine + 1 mL (100 μg) of dexmedetomidine. Anaesthesiologist involved in the data collection as well as the patients were blinded to the content of the study solution. The preparation of the study drug solutions were done by an anaesthesiologist not involved in the study.
Pre operative procedure: Preoperatively, an 18G IV line was placed in the non-surgical hand and ringer lactate was initiated at 5 mL/kg/hr. On arrival to the operation theater, ASA recomended standard monitors like pulse oximeter, non-invasive blood pressure and electrocardiograph (ECG) were attached, and baseline readings were taken. HR, SBP and DBP were recorded at 0 min and every 5 minutes for the first 15 minutes, then 15 minutes once for the first hour and every thirty minutes thereafter till the end of surgery. The patients were positioned supine with head extended and turned towards opposite side and the arm was adducted and fully extended towards the ipsilateral knee as much as possible. The midpoint of clavicle was marked and patients were told to raise the head slightly and posterior border of sternocleidomastoid was palpated and further proceeded backwards to pass over anterior scalene muscle into the inter scalene groove. The meeting point of a mark made 1.5 to 2.0 cm from the midpoint of clavicle intersecting the interscalene groove denotes the location of subclavian artery confirming the landmark for needle entry [12]. Neural localization was achieved using a nerve stimulator (Stimuplex; DIG Nerve stimulator, B. Braun, Germany) connected to a stimulating needle (Stimuplex Needle, B Braun, Germany 22 gauge, 4 cm length). The appropriate position of the needle in relation to the plexus was achieved when a current of <0.5 mA (miliamperes) produced minimal distal motor response. On localisation of the brachial plexus, a total volume of 40 mL of local anaesthetic mixture was injected incrementally after negative aspiration for blood or air.
Sensory block was examined by pinprick test with a 3-point scale in all nerve territories: 0-sharp pin felt, 1-dull sensation felt (analgesia), 2-no sensation felt (anaesthesia).
Motor block was assessed by thumb abduction, adduction and opposition corresponding to radial, ulnar and median nerves respectively. Musculocutaneous nerve was assessed by flexion at the elbow joint. The overall motor function was assessed on a 3-point scale depicting 0-normal flexion and extension of elbow, wrist and fingers, 1-reduced motor strength but could move fingers, 2-complete motor blocks with inability to move fingers.
Sensory and motor blockade were assessed every three minutes for the first 30 minutes after the drug injection, every 30 minutes during the intraoperative period and then every hour until they had resolved. Onset of sensory block was defined as the time between the end of local anaesthetic administration to complete sensory block. Complete sensory block is defined as anaesthetic blockade with score 2 on the 3-point sensory assessment scale on all nerve territories. Duration of sensory block is stated as the time span between the end of local anaesthetic administration to complete resolution of anaesthesia in all nerve distributions [13]. Onset of motor block is the time interval existing between end of local anaesthetic administration to loss of movements. Complete motor block is defined by absence of voluntary movements in the hand and forearm (score 2). Duration of motor block is stated as the time between completion of local anaesthetic administration to recovery of complete motor function of the hand and forearm [13]. Duration of post operative analgesia is taken as the time interval existing between completion of local anaesthetic administration to the first request for rescue analgesia.
After completion of 30 minutes if the desired sensory and motor levels of block were not achieved, then the block was considered as a failure and the patients were excluded from the study and general anaesthesia was administered.
Adverse events such as hypotension (drop in SBP by 20% from baseline) was treated with Inj.Mephentermine 6 mg IV. Bradycardia (decrease in heart rate less than 50 beats per minute) was treated with Inj Atropine 0.6 mg IV bolus. Hypoxia with SPO2 <90% was treated with oxygen by mask.
Statistical Analysis
For statistical analysis, the software Statistical Package for the Social Sciences (SPSS) version 20.0® for Windows was used. All the data were expressed as Mean±Standard Deviation (SD). Quantitative data was compared using student’s unpaired t-test while qualitative data was compared using Chi-square test. The p-value of <0.05 was considered statistically significant.
Results
Fifty patients were randomly divided into two groups (LS and LD). The groups were comparable with respect to their demographic profiles like age, sex distribution and weight [Table/Fig-2].
Demographic profile of patients.
| Variables | Group LS | Group LD | p-value |
|---|
| Mean age (in years) | 41.7±14.71 | 40.7±15.83 | 0.81 |
| Male to Female ratio | 18:7 | 16:9 | 0.54 |
| Mean weight (kg) | 66.5±10.16 | 65.4±12.03 | 0.72 |
LS: Levobupivacaine with saline, LD: Levobupivacaine with dexmedetomidine
Chi-square test was used
The mean time for onset of sensory and motor blockade was earlier in group LD than in group LS. The statistical analysis by students unpaired t-test revealed that a significant difference existed in the mean onset times of sensory and motor block between the two groups with p-value <0.001. The mean duration of sensory and motor blockade was also significantly prolonged in group LD compared to group LS with p-value <0.001 [Table/Fig-3].
Onset and duration of sensory and motor blockade with duration of analgesia.
| Variables | Group LS Mean+SD | Group LD Mean+SD | p-value |
|---|
| Onset of sensory block (min) | 8±0.76 | 5±0.73 | <0.001* |
| Onset of motor block (min) | 17.4±1.26 | 14±1.33 | <0.001* |
| Duration of sensory block (min) | 759.6±37.24 | 838.8±42.35 | <0.001* |
| Duration of motor block (min) | 667.2±42.67 | 738±45.64 | <0.001* |
| Duration of analgesia (h) | 14.3±0.61 | 16.3±0.64 | <0.001* |
LS: Levobupivacaine with saline; LD: Levobupivacaine with dexmedetomidine; *p<0.05 Students Unpaired t-test was used
The mean duration of analgesia was greatly prolonged in group LD when comparison to group LS. This difference between the two groups was highly significant with p-value <0.001 which proves that adding dexmedetomidine to levobupivacaine significantly increases analgesic duration in brachial plexus blockade via supraclavicular approach [Table/Fig-3].
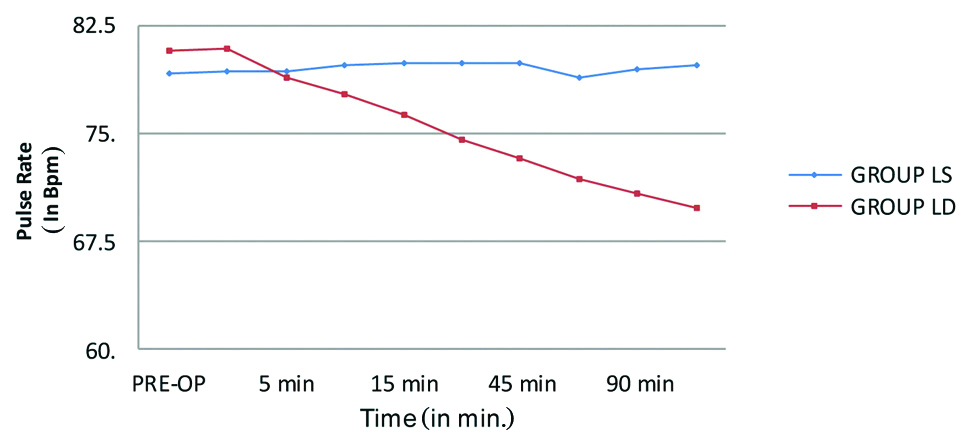
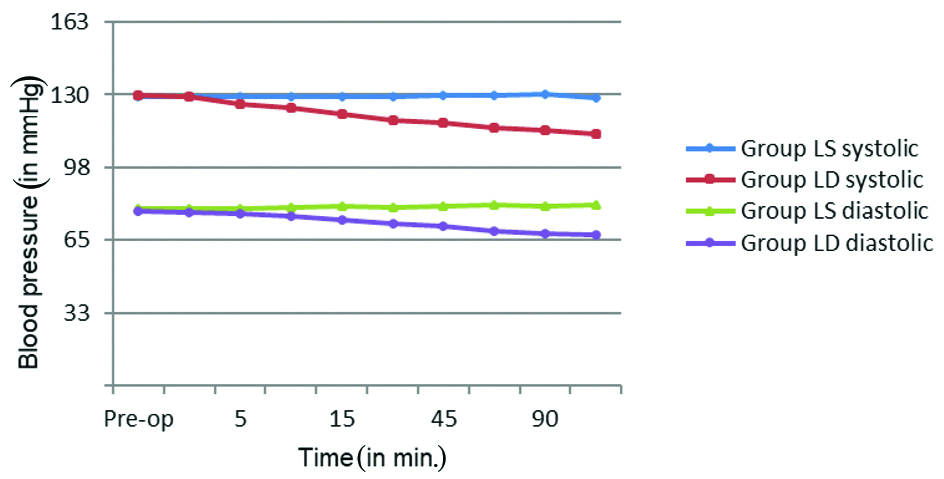
The intraoperative mean pulse rate was comparable between the groups until the first 15 minutes. A steady decline in the mean pulse rate was seen in group LD compared to group LS at 30 minutes and at different time intervals thereafter during the intraoperative period and the difference was statistically significant with p-value <0.001. Similarly, the mean SBP and DBP between the groups were comparable until the first 10 minutes intraoperatively then a steady decline in the mean SBP and DBP was seen in group LD compared to group LS at 15 minutes and thereafter during the intraoperative period which was also statistically significant with a p-value <0.001. These haemodynamic changes are represented in the [Table/Fig-4,5]. There were no other block or procedure related complications or side effects that were encountered in either of the groups during the study.
Pulse rate changes across the group.
LS: Levobupivacaine with saline; LD: Levobupivacaine with dexmedetomidine
Blood pressure changes across the group.
LS: Levobupivacaine with saline; LD: Levobupivacaine with dexmedetomidine
Discussion
Brachial plexus blockade has been traditionally employed as an anaesthetic modality of choice for performing upper limb surgeries. Nevertheless, the supraclavicular approach to the plexus is the commonest way of approaching the trunks where the collaboration of sensory, motor and sympathetic innervation exists in a very small surface area, eventually culminating in a quick, precise and superior anaesthetic blockade with excellent success rates [14]. Levobupivacaine is a good substitute for bupivacaine as it not only provides longer duration of sensory blockade but is also associated with good analgesia with minimal systemic side effects [15]. Dexmedetomidine, an α2-adrenergic agonist is being commonly used as an adjuvant to local anaesthetic because of its sedative, analgesic, anti-hypertensive, anti-emetic actions in addition to reducing the anaesthetic drug requirements [16].
In the present study, group LS received 39 mL of 0.5% levobupivacaine + 1 mL normal saline and group LD received 39 mL of 0.5% levobupivacaine + 1 mL (100 μg) of dexmedetomidine. The mean onset time for sensory and motor blockade was significantly shorter in group LD than in group LS. The results of this study are similar to a study conducted by Kaur H et al., who observed that the time of onset for sensory and motor blockade were 6.9 and 7.6 minutes in dexmedetomidine group compared to 7.6 and 8.3 minutes in the control group respectively [17]. Levobupivacaine results in conduction blockade by inhibiting the passage of sodium ions through ion-selective sodium channels in the nerve membranes thereby terminating nerve impulse transmission [18,19]. The addition of an α-2 agonist like dexmedetomidine to local anaesthetics induces vasoconstriction in and around the administration site eventually leading to a delay in absorption of the local anaesthetic thereby providing increased volume of levobupivacaine at the site of action [20,21]. Dexmedetomidine reduces the release of norepinephrine in the peripheral adrenoceptors and mediates an α2-receptor independent inhibitory effect on nerve fibre action potentials [22]. The different mechanism of action of both the drugs can have an additive effect and hence when mixed together shortens the onset of sensory and motor blockade.
The mean time for duration of sensory and motor blockade was significantly longer in group LD than in group LS. The prolonged duration in the dexmedetomidine group observed in this study were similar to the results of Biswas S et al., where duration of sensory block in the dexmedetomidine group was 898 minutes compared to 645 minutes observed in the control group and the duration of motor block was 840 minutes in dexmedetomidine group against 512 minutes in the control group which was statistically significant [23].
The mean duration of analgesia in the present study appeared to be significantly longer in group LD than in group LS (p-value <0.001). These findings were similar to a study done by Esmaoglu A et al., who observed a significantly prolonged analgesic duration in dexmedetomidine group in comparison to the control group [2]. Dexmedetomidine not only terminates the pain signals by suppressing the activity in the descending noradrenergic pathway which in turn modulates nociceptive neurotransmission. It also inhibits the release of substance P at the level of the dorsal root neurons and activates α2-adrenergic receptors in the locus coeruleus which can provide prolonged analgesia [24,25].
A specific change in trend of vital parameters was observed in this study. The mean pulse rate, systolic and diastolic blood pressure was comparable between the groups until the first 15 minute of intraoperative period but there was a significant change in the vital parameters in dexmedetomidine group from 30 minutes of intraoperative period until the completion of the study. Dexmedetomidine inhibits sympathetic activity by post-synaptic activation of α2-receptors thereby decreasing HR and BP. Persistence of bradycardia is attributed to central sympathetic inhibition. The normal baraoreceptor response and HR reflex to a vasopressor agent is however preserved with the use of dexmedetomidine thereby conferring feasibility to clinically tackle and treat hypotension and bradycardia providing haemodynamic control [26]. A similar observation with lower mean HR, SBP, and DBP in the dexmedetomidine group was also encountered by Agarwal S et al., on evaluating the efficacy of adding dexmedetomidine to bupivacaine in supraclavicular brachial plexus block [27]. Hence, the present study established that levobupivacaine with dexmedetomidine reduces the onset time and enhances the duration of sensory and motor blockade along with increased duration of analgesia without any side-effects.
Limitation(s)
The limitation of the study was the usage of standard dose of dexmedetomidine (100 mcg) for the study group as there was no weight based dosing available for peripheral nerve blockade.
Conclusion(s)
Dexmedetomidine shortens the onset time and greatly prolongs the duration of sensory and motor blockade effectively enhancing the quality of anaesthesia, when employed as an adjuvant for upper limb anaesthetic blockades. It is also an adjuvant with greatest potential in prolonging the duration of analgesia with minimal systemic side-effects for peripheral nerve blockades. Further research should be targeted in other combinations of local anaesthetics with dexmedetomidine and employing addition of adjuvant in the control arm as well to provide the benefit of prolonged anaesthesia for both the groups for better clinical comparison and anaesthetic outcomes.
LS: Levobupivacaine with saline, LD: Levobupivacaine with dexmedetomidine
Chi-square test was used
LS: Levobupivacaine with saline; LD: Levobupivacaine with dexmedetomidine; *p<0.05 Students Unpaired t-test was used