Introduction
Owing to the lack of any standard treatment, subjective tinnitus can be debilitating, manifesting a varied population response to tinnitus management. There is, therefore, an unmet need to optimise the existing treatment options and generate data to help physicians provide the best possible care for individual patients.
Aim
To compare the effectiveness and safety of Ginkgo biloba, vinpocetine, and piracetam as a single agent and their Fixed Dose Combination (FDC) in patients with subjective tinnitus.
Materials and Methods
Patients with complaints of subjective tinnitus were enrolled in this longitudinal cohort, single centre study which was conducted at Outpatient Clinic of the ENT Department of Justice KS Hegde Charitable Hospital, Karnataka, India. Patients received one of the five treatments, oral route, three times a day, {group 1: Ginkgo biloba (40 mg); group 2: vinpocetine (5 mg); group 3: piracetam (400 mg); group 4: FDC of Ginkgo biloba (60 mg) and piracetam (400 mg); group 5: FDC of Ginkgo biloba (60 mg), piracetam (800 mg) and vinpocetine (5 mg)} and were followed-up for six weeks using a modified version of Tinnitus Handicap Inventory (THI) and Visual Analogue Scale (VAS), before and after the treatment. Data for safety were also recorded. The association between each attribute and the presence of tinnitus was assessed through chi-square tests.
Results
A total of 130 out of 149 enrolled patients completed the study. All the groups showed significant improvement in the severity of symptoms at the end of six weeks as assessed by the modified THI and VAS scores. The improvement was found to be better in group 5 than in other groups, which was evident from the percentage improvement at the end of the treatment compared to other groups. No adverse drug reactions were associated with any of the treatment groups.
Conclusion
Though all the drugs were found to be effective and safe in reducing the intensity of subjective tinnitus, FDC of Ginkgo biloba-piracetam-vinpocetine may be considered a better alternative than Ginkgo biloba-piracetam combination and Ginkgo biloba, piracetam, or vinpocetine as single agents.
Introduction
Tinnitus is the perceptional expression of any sound in the absence of a corresponding external acoustic stimulus. Tinnitus may be rhythmical or pulsatile, constant or intermittent, localised to one or both ears, centrally within the head, abrupt or insidious in onset, and temporary or longstanding [1]. The aetiology of tinnitus remains unknown, but some underlying clinical causes such as otologic, neurologic, infectious, nasal allergies, autoimmunity, cardiovascular diseases, diabetes, and degenerative neural disorders with socio-demographic and environmental factors, have been reported [2,3].
Population based evidence indicates that 5-32% of the world population is affected by tinnitus [4-9], with 6% having a severe degree of tinnitus in the United States [10]. However, the inconsistency in defining and reporting tinnitus, leading to variability in estimates, should be considered. The enigma of tinnitus in India is also as alarming and as severe as in the rest of the world; unfortunately, there are no exact data available on the prevalence and aetiology of tinnitus, except for few isolated studies [2,10]. Moreover, many people habituate to the phantom sound and choose not to seek medical treatment.
Tinnitus can be objective or subjective. Objective tinnitus, also termed as “pseudo-tinnitus,” “vibratory,” or “extrinsic,” is either due to vascular phenomena or muscle changes such as spasm of the muscle of the middle ear or palate [10]. However, subjective tinnitus is much more common and results from abnormal neural activities which are not formed by sounds [11]. In subjective tinnitus, the neural signals corresponding to the tinnitus present in the auditory cortex may have been produced by a lesion in the cortex itself or at any further stage in the auditory pathway. Thus, subjective tinnitus may arise within the cochlea or in the subsequent phases of the auditory system [12]. Often, tinnitus is present in patients without any detectable abnormality in the external, middle, or inner ear or the auditory nerve.
Tinnitus may significantly impact quality of life, with debilitating depression, anxiety, frustration and insomnia, and decreased speech discrimination. Functional impairment in thought processing, emotions, hearing, and concentration are also often observed [13]. A survey by Tyler RS and Baker LJ first identified the wide range of effects of tinnitus on the quality of life of the patient [14]. Numerous other studies, with similar results, have documented the wide range of difficulties faced by those with bothersome tinnitus [15-17]. However, due to tinnitus’s heterogeneity in aetiology, pathophysiology, and clinical characteristics, tinnitus remains a scientific and clinical enigma, exacerbating variable population response to tinnitus management.
There is no successful drug treatment available, although much research is underway on mechanisms and possible therapies. The most commonly used treatment modes for tinnitus are medical treatment, surgical treatment, palliative treatment, use of tinnitus maskers/hearing aid if there is accompanying hearing loss, electrical stimulation, psychotherapy, relaxation therapy, etc., but with little success [18].
Hence, there is an unmet need to optimise current treatment options and generate data to help physicians provide the best possible care for individual patients. The objective of this study was to compare the effectiveness of Ginkgo biloba, vinpocetine, and piracetam as single agents and as a FDC, which was the commonly employed treatment modality in a tertiary care set-up in the Southern India for the treatment of tinnitus.
Materials and Methods
The present study was a longitudinal cohort, single-centre study which was conducted between January 2013 to January 2014. The study was approved by the Institutional Ethics Committee (INST.EC/E.C/66/2012-13). Written informed consent was obtained from each patient before the enrollment.
Inclusion and exclusion criteria: Patients aged ≥18 years with complaints of subjective tinnitus, who visited the Outpatient Clinic of the ENT Department of Justice KS Hegde Charitable Hospital, Karnataka, India, were included in the study. Patients on anticoagulants, undergoing psychiatric treatment, with abnormal blood pressure, or any condition deemed inappropriate for inclusion as per the physician were excluded from the study. Pregnant or lactating women were also excluded from the study.
Treatment
All patients presenting with subjective tinnitus were randomly allocated to one of the following five treatment groups (1:1:1:1:1 ratio), oral route, three times a day for six weeks, based on the treating physician’s discretion.
Group 1- Ginkgo biloba 40 mg
Group 2- Vinpocetine 5 mg
Group 3- Piracetam 400 mg
Group 4- FDC of Ginkgo biloba 60mg and piracetam 400 mg
Group 5- FDC of Ginkgo biloba 60 mg, piracetam 800 mg and vinpocetine 5 mg
The patients took the first dose on the day following dose allocation. A simple randomisation technique with a unique number was followed. During the treatment period, patients continued the assigned study treatment. Considering the effect size of 0.5, a 2-sided type I error rate of 5%, and a statistical power of 80%, the estimated sample size was calculated to be 149 in total.
All patients were administered the modified version of the Tinnitus Handicap Inventory (THI) before and after the treatment [19]. This is a questionnaire-based test that Ear, Nose and Throat physicians and audiologists use to determine the tinnitus patient’s degree of distress. The modified questionnaire consisted of five questions [Appendix 1]. For each question, the patient responded with either a “yes” (4 points), “sometimes” (2 points), or “no” (0 points). Total score was then noted before and after the treatment. Similarly, tinnitus was measured both before and after treatment using a VAS with a range of 0 to 10, with 0 corresponding to no tinnitus and 10 to unbearable tinnitus [20].
Secondary safety endpoints included the frequency and severity of adverse reactions.
Statistical Analysis
Descriptive statistics were used to analyse the study results. The continuous variables were presented as Mean±SD, and the categorical variables as frequencies and percentages. The association between each attribute and the presence of tinnitus was assessed through Chi-square tests. A p-value <0.05 was considered as a statistical level of significance. All the statistical analyses were performed using Statistical Analysis System (SAS) software version 9.4.
Results
A total of 130 out of 149 enrolled patients completed the study (26 patients in each group). Out of 31 patients in group 1, three patients were lost to follow-up, and two became pregnant. Out of 29 patients in group 2, three were lost to follow-up. Out of 30 patients in group 3, one became pregnant, and three were lost to follow-up. A total of four out of 30 patients in group 4 and three out of 29 patients in group 5 were lost to follow-up [Table/Fig-1].
Patient flow diagram.
FU: Follow-up
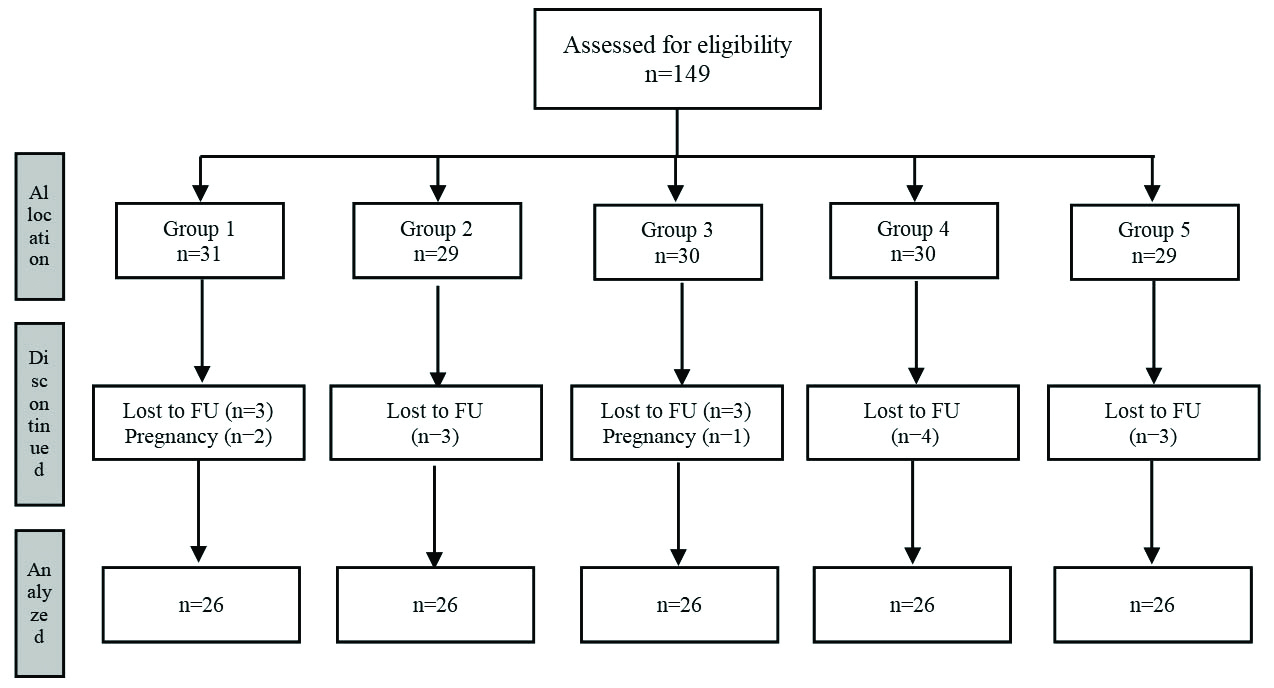
The baseline characteristics were comparable between the groups. The mean age of the population was 52.38 (14.28) years. There was an equal distribution of women and men in the study (n=65 each). There was no significant difference between the groups with respect to age (χ2=1.632; p=0.170) and gender (χ2=2.154; p=0.707) [Table/Fig-2].
Baseline characteristics.
| Parameter | Group | p-value |
|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|
| Age (years), Mean (SD) | 50.85 (13.58) | 58.31 (14.08) | 52.85 (15.89) | 49.38 (14.14) | 50.50 (12.84) | 0.170 |
| Gender, n (%) |
| Female | 13 (20.0%) | 10 (15.4%) | 15 (23.1%) | 13 (20.0%) | 14 (21.5%) | 0.707 |
| Male | 13 (20.0%) | 16 (24.6%) | 11 (16.9%) | 13 (20.0%) | 12 (18.5%) |
| Ear pathology, n (%) |
| Chronic suppurative otitis media | 0 | 2 (7.7%) | 3 (11.5%) | 4 (15.4%) | 6 (23.1%) | 0.192 |
| Meniere’s | 3 (11.5%) | 1 (3.8%) | 2 (7.7%) | 4 (15.4%) | 2 (7.7%) | 0.24 |
| Noise induced sensorineural hearing loss | 2 (7.7%) | 7 (26.9%) | 4 (15.4%) | 5 (19.2%) | 4 (15.4%) | 0.08 |
| Presbycusis | 5 (19.2%) | 3 (11.5%) | 2 (7.7%) | 3 (11.5%) | 3 (11.5%) | 0.12 |
| Idiopathic tinnitus | 16 (61.5%) | 13 (50.0%) | 15 (57.7%) | 10 (38.5%) | 11 (42.3%) | 0.16 |
| Nature of Tinnitus, n (%) |
| Continuous | 12 (20.0%) | 11 (18.3%) | 12 (20.0%) | 14 (23.3%) | 11 (18.3%) | 0.920 |
| Intermittent | 14 (20.0%) | 15 (21.4%) | 14 (20.0%) | 12 (17.1%) | 15 (21.4%) | |
| Onset of Tinnitus, n (%) |
| Insidious | 13 (15.7%) | 14 (16.9%) | 20 (24.1%) | 17 (20.5%) | 19 (22.9%) | 0.185 |
| Sudden | 13 (27.7%) | 12 (25.5%) | 6 (12.8%) | 9 (19.1%) | 7 (14.9%) |
| Duration of symptoms (months), Mean (SD) | 13.67 (21.016) | 10.60 (17.24) | 5.11 (6.66) | 6.41 (12.86) | 7.75 (15.61) | 0.0277 |
| Co-morbidities |
| Diabetes mellitus | 3 (15.8%) | 4 (21.1%) | 5 (26.3%) | 3 (15.8%) | 4 (21.1%) | 0.06 |
| Hypertension | 5 (18.5%) | 6 (22.2%) | 6 (22.2%) | 4 (14.8%) | 6 (22.2%) | 0.1 |
Chi-square test was used for calculating p-value
A statistically significant improvement in tinnitus was observed in all the groups at the end of the treatment (six weeks) compared to baseline, as evident from the modified THI and VAS score (p<0.001; [Table/Fig-3]). The improvement was found to be better in patients on FDC, and more so in patients in group 5 compared to other groups [Table/Fig-3]. The inter-group differences were significant (p<0.001).
Score at baseline and at the end of treatment (Full Analysis Set).
| Score | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | p-value* |
|---|
| At baseline | After six weeks | Change from baseline | At baseline | After six weeks | Change from baseline | At baseline | After six weeks | Change from baseline | At baseline | After six weeks | Change from baseline | At baseline | After six weeks | Change from baseline |
|---|
| Custo-mised THI score, Mean (SD) | 15.46 (2.75) | 10.23 (2.42) | 5.23 (3.491) | 15.62 (2.77) | 10.38 (2.4) | 5.23 (3.798) | 15.54 (2.49) | 10.54 (2.5) | 5.00 (3.359) | 16 (2.71) | 5.62 (4.45) | 10.38 (5.879) | 15.38 (2.45) | 3.31 (3.39) | 12.08 (4.251) | <0.001 |
| VAS score, (SD) | 8.35 (1.09) | 6.58 (1.39) | 1.77 (1.966) | 8.5 (0.81) | 6.42 (1.45) | 2.08 (1.262) | 8.31 (0.88) | 6.65 (1.02) | 1.65 (1.413) | 8.46 (0.9) | 2.5 (1.86) | 5.96 (2.107) | 8.54 (0.86) | 1.54 (1.17) | 7.00 (1.386) | <0.001 |
*p-value denotes change from baseline; Chi-square test was used
Safety: No adverse reactions were associated with any of the treatment groups.
Discussion
In present study, the mean age of patients presenting with tinnitus was found to be 52.38 years. This was in line with the published evidence, which indicates that the prevalence of chronic tinnitus increases with increasing age [21], most of them between the ages of 40 and 80 years [22]. The increase in tinnitus prevalence with age is partly explained by the fact that hearing loss is a significant risk factor for tinnitus, and hearing loss prevalence also increases with age [6,23]. The prevalence of tinnitus rises to 70–85% in the hearing-impaired population; 40 to 60 years old individuals are most affected, followed by individuals above 60 years of age, followed by individuals below 40 years of age [22,24-25].
Studies have been disparate on gender preponderance in tinnitus. Although some have described a slightly higher prevalence in women [9], others have suggested that the prevalence is higher in men [26-28]. In this study, an equal predilection for men and women was noted, as was evident in the recruitment period.
Gudwani S et al., in their study, observed that the majority of tinnitus ears (72%) had onset duration longer than six months [29]. In present study, the mean duration of tinnitus was found to be approximately eight months. In subjective tinnitus, as there are no objective measures, the patient’s description of their symptoms is the only source of information. Hence, in addition to taking a detailed case history and conducting otological and audiological assessments, tinnitus questionnaires are used to assess the levels of tinnitus-related distress and tinnitus handicap and the patient’s emotional reactions.
Though various studies have been done to understand the effect of Ginkgo biloba, vinpocetine, and piracetam in tinnitus, the literature is scarce and varied. The herb Ginkgo biloba is often used to help manage tinnitus because of its vasodilating and antioxidant properties. But studies are small and have conflicting results [30-37]. This inconsistency could be attributed to the methodological differences, quality, and dosage of Ginkgo biloba. Nevertheless, Ginkgo biloba is frequently suggested as a potential treatment for tinnitus, and many people with tinnitus are using a diversity of products based on limited evidence.
Some early research suggests that giving vinpocetine along with physiotherapy is useful in the treatment of tinnitus. A retrospective study conducted between 2001-2006 on patients suffering from chronic unilateral or bilateral tinnitus compared the different treatment modalities to define their effectiveness for tinnitus relief. The treatment consisted of the use of pentoxifylline, lidocaine, or vinpocetine alone, intravenously, or in combination with physiotherapy and soft laser therapy. A combination of vinpocetine and physiotherapy was found to be the most effective treatment for tinnitus in these patients [38]. The utility of vinpocetine in the treatment of acoustic trauma with subsequent hearing loss and tinnitus was studied by Ribári O et al., [39]. Disappearance of tinnitus occurred in 50% of those who started vinpocetine within one week of the trauma. A total of 79% of patients had better hearing since the incident; 66% had a substantial improvement in the incidence of tinnitus [39].
In a clinical study on 39 patients with tinnitus and sudden hearing loss, piracetam was compared with that of naftidrofuryl, with respect to hearing improvement and the reduction in the intensity of tinnitus. The two groups were comparable in terms of demographic and audiological baseline data. The improvement in tinnitus was found to be 27 dB with piracetam and 19.9 dB with naftidrofuryl. Piracetam, which enhanced rheology and impacted metabolism positively, was considered effective for acute tinnitus treatment [40].
Subjective tinnitus is experienced in different forms, with a wide range of severity and character. In present study, the patients in all five groups were comparable at baseline in tinnitus intensity. Present study data showed that Ginkgo biloba, vinpocetine, and piracetam, when used alone as a single agent, produced an overall reduction in tinnitus intensity compared to baseline (p<0.01).
The FDC showed a more significant reduction in tinnitus intensity compared to the drugs when used individually. Further, the improvement was better in patients on the FDC of Ginkgo biloba-piracetam-vinpocetine than in other groups.
Further, in present study, Ginkgo biloba, piracetam, and vinpocetine were found to be safe and tolerable in these patients with subjective tinnitus. None of the patients complained of any adverse reactions. This was consistent with the published literature [40-43]. Since tinnitus is multifactorial, a multimodal approach to tinnitus treatment employing FDCs may help bring more relief to many human beings who suffer alone, plagued by the sounds of silence. However, it is recommended to evaluate patients individually at a more extensive level in future studies.
Limitation(s)
The present study did not consider the placebo effect, and therefore it is not possible to distinguish the effect produced by these drugs from the placebo effect. Additionally, the modified questionnaire used in the study was not validated. Hence further studies with a validated questionnaire on a larger population are warranted to substantiate present study findings.
Conclusion(s)
When used as single agents, Ginkgo biloba, vinpocetine, or piracetam resulted in significant improvement in their symptoms compared to baseline. The study results also indicated that FDC of Ginkgo biloba-piracetam and Ginkgo biloba-piracetam-vinpocetine are potent treatment options in these patients. Moreover, out of the two FDCs studied, Ginkgo biloba with piracetam and vinpocetine may be considered a better alternative than Ginkgo biloba-piracetam combination and Ginkgo biloba, piracetam or vinpocetine as single agents. Ginkgo biloba, piracetam, and vinpocetine demonstrated a favourable safety profile in these patients.
Chi-square test was used for calculating p-value
*p-value denotes change from baseline; Chi-square test was used
[1]. Baguley D, McFerran D, Hall D, TinnitusLancet 2013 382(9904):1600-07.10.1016/S0140-6736(13)60142-7 [Google Scholar] [CrossRef]
[2]. Manche SK, Madhavi J, Meganadh KR, Jyothy A, Association of tinnitus and hearing loss in otological disorders: A decade-long epidemiological study in a South Indian populationBraz J Otorhinolaryngol 2016 82(6):643-49.10.1016/j.bjorl.2015.11.00726923827 [Google Scholar] [CrossRef] [PubMed]
[3]. Han BI, Lee HW, Kim TY, Lim JS, Shin KS, Tinnitus: Characteristics, causes, mechanisms, and treatmentsJ Clin Neurol 2009 5(1):11-19.10.3988/jcn.2009.5.1.1119513328 [Google Scholar] [CrossRef] [PubMed]
[4]. Heller AJ, Classification and epidemiology of tinnitusOtolaryngol Clin North Am 2003 36(2):239-48.10.1016/S0030-6665(02)00160-3 [Google Scholar] [CrossRef]
[5]. Quaranta A, Assennato G, Sallustio V, Epidemiology of hearing problems among adults in ItalyScand Audiol Suppl 1996 42:09-13. [Google Scholar]
[6]. Pilgramm M, Rychlick R, Lebisch H, Siedentop H, Goebel G, Kirchhoff D, Tinnitus in federal republic of Germany: A representative epidemiological study. In: Hazell J, editorProceedings of the 6th International Tinnitus Seminar 1999 Cambridge, UK:64-67. [Google Scholar]
[7]. Johansson MS, Arlinger SD, Prevalence of hearing impairment in a population in SwedenInt J Audiol 2003 42:18-28.10.3109/1499202030905608112564512 [Google Scholar] [CrossRef] [PubMed]
[8]. Mercier V, Luy D, Hohmann BW, The sound exposure of the audience at a music festivalNoise Health 2003 5:51-58. [Google Scholar]
[9]. Axelsson A, Ringdahl A, Tinnitus- A study of its prevalence and characteristicsBr J Audiol 1989 23:53-62.10.3109/030053689090778192784987 [Google Scholar] [CrossRef] [PubMed]
[10]. Makar SK, Biswas A, Shatapathy P, The impact of tinnitus on sufferers in Indian populationIndian J Otolaryngol Head Neck Surg 2014 66(Suppl 1):37-51.10.1007/s12070-011-0291-x24533358 [Google Scholar] [CrossRef] [PubMed]
[11]. McCormack A, Edmondson-Jones M, Fortnum H, Dawes P, Middleton H, Munro KJ, The prevalence of tinnitus and the relationship with neuroticism in a middle-aged UK populationJ Psychosom Res 2014 76(1):56-60.10.1016/j.jpsychores.2013.08.01824360142 [Google Scholar] [CrossRef] [PubMed]
[12]. Haider HF, Bojic T, Ribeiro SF, Paço J, Hall DA, Szczepek AJ, Pathophysiology of subjective tinnitus: Triggers and maintenanceFront Neurosci 2018 12:86610.3389/fnins.2018.0086630538616 [Google Scholar] [CrossRef] [PubMed]
[13]. Nondahl DM, Cruickshanks KJ, Dalton DS, Klein BEK, Klein R, Schubert CR, The impact of tinnitus on quality of life in older adultsJ Am Acad Audiol 2007 18(3):257-66.10.3766/jaaa.18.3.717479618 [Google Scholar] [CrossRef] [PubMed]
[14]. Tyler RS, Baker LJ, Difficulties experienced by tinnitus sufferersJ Speech Hear Disord 1983 48:150-54.10.1044/jshd.4802.1506621006 [Google Scholar] [CrossRef] [PubMed]
[15]. Lasisi AO, Gureje O, Prevalence of insomnia and impact on quality of life among community elderly subjects with tinnitusAnn Otol Rhinol Laryngol 2011 120:226-30.10.1177/00034894111200040221585151 [Google Scholar] [CrossRef] [PubMed]
[16]. Cima RF, Crombez G, Vlaeyen JW, Catastrophizing and fear of tinnitus predict quality of life in patients with chronic tinnitusEar Hear 2011 32:634-41.10.1097/AUD.0b013e31821106dd21399500 [Google Scholar] [CrossRef] [PubMed]
[17]. Stouffer JL, Tyler RS, Characterization of tinnitus by tinnitus patientsJ Speech Hear Disord 1990 55(3):439-53.10.1044/jshd.5503.4392381186 [Google Scholar] [CrossRef] [PubMed]
[18]. Soleymani T, Pieton D, Pezeshkian P, Miller P, Gorgulho AA, Pouratian N, Surgical approaches to tinnitus treatment: A review and novel approachesSurg Neurol Int 2011 2:15410.4103/2152-7806.8683422140639 [Google Scholar] [CrossRef] [PubMed]
[19]. Newman CW, Sandridge SA, Bolek L, Development and psychometric adequacy of the screening version of the tinnitus handicap inventoryOtol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol 2008 29(3):276-81.10.1097/MAO.0b013e31816569c418277308 [Google Scholar] [CrossRef] [PubMed]
[20]. Torrance GW, Feeny D, Furlong W, Visual analog scales: Do they have a role in the measurement of preferences for health states?Med Decis Making 2001 21:329-34.10.1177/0272989012206262211475389 [Google Scholar] [CrossRef] [PubMed]
[21]. Shargorodsky J, Curhan SG, Curhan GC, Eavey R, Change in prevalence of hearing loss in US adolescentsJAMA 2010 304:772-78.10.1001/jama.2010.112420716740 [Google Scholar] [CrossRef] [PubMed]
[22]. Al-Swiahb J, Park SN, Characterization of tinnitus in different age groups: A retrospective reviewNoise Health 2016 18(83):214-19.10.4103/1463-1741.18924027569409 [Google Scholar] [CrossRef] [PubMed]
[23]. Fioretti A, Poli O, Varakliotis T, Eibenstein A, Hearing disorders and sensorineural agingJournal of Geriatrics 2014 10.1155/2014/602909 [Google Scholar] [CrossRef]
[24]. Meikle M, Walsh T, Characteristics of tinnitus and related observations in over 1800 tinnitus clinic patientsJ Laryngol Otol 1984 (Suppl 9):17-21.10.1017/S17551463000900536596358 [Google Scholar] [CrossRef] [PubMed]
[25]. Schlee W, Kleinjung T, Hiller W, Goebel G, Kolassa IT, Langguth B, Does tinnitus distress depend on age of onset?PLoS One 2011 6(11):e2737910.1371/journal.pone.002737922125612 [Google Scholar] [CrossRef] [PubMed]
[26]. Maas IL, Brüggemann P, Requena T, Bulla J, Edvall NK, Hjelmborg JVB, Genetic susceptibility to bilateral tinnitus in a Swedish twin cohortGenet Med 2017 19(9):1007-12.10.1038/gim.2017.428333916 [Google Scholar] [CrossRef] [PubMed]
[27]. Holgers KM, Zoger S, Svedlund K, Predictive factors for development of severe tinnitus suffering further characterizationInt J Audiol 2005 44(10):584-92.10.1080/1499202050019023516315449 [Google Scholar] [CrossRef] [PubMed]
[28]. Lockwood AH, Salvi RJ, Burkard RF, TinnitusN Engl J Med 2002 347(2):904-10.10.1056/NEJMra01339512239260 [Google Scholar] [CrossRef] [PubMed]
[29]. Gudwani S, Munjal SK, Panda NK, Verma RK, Correlation of tinnitus loudness and onset duration with audiological profile indicating variation in prognosisISRN Otolaryngology 2013 2013:20571410.1155/2013/20571424078882 [Google Scholar] [CrossRef] [PubMed]
[30]. Ernst E, Stevinson C, Ginkgo biloba for tinnitus: A reviewClin Otolaryngol 1999 24:164-67.10.1046/j.1365-2273.1999.00243.x10384838 [Google Scholar] [CrossRef] [PubMed]
[31]. von Boetticher A, Ginkgo biloba extract in the treatment of tinnitus: A systematic reviewNeuropsychiatr Dis Treat 2011 7:441-47.10.2147/NDT.S2279321857784 [Google Scholar] [CrossRef] [PubMed]
[32]. Drew S, Davies E, Effectiveness of Ginkgo biloba in treating tinnitus: Double blind, placebo controlled trialBMJ 2001 322(7278):01-06.10.1136/bmj.322.7278.7311154618 [Google Scholar] [CrossRef] [PubMed]
[33]. Rejali D, Sivakumar A, Balaji N, Ginkgo biloba does not benefit patients with tinnitus: a randomised placebo-controlled double-blind trial and meta-analysis of randomised trialsClin Otolaryngol Allied Sci 2004 39(3):226-31.10.1111/j.1365-2273.2004.00814.x15142066 [Google Scholar] [CrossRef] [PubMed]
[34]. Smith PF, Zheng Y, Darlington CL, Ginkgo biloba extracts for tinnitus: More hype than hope?J Ethnopharmacol 2005 100(1-2):95-99.10.1016/j.jep.2005.05.03215998570 [Google Scholar] [CrossRef] [PubMed]
[35]. Hilton MP, Stuart EL, Ginkgo biloba for tinnitusCochrane Database Syst Rev 2004 2:CD00385210.1002/14651858.CD003852.pub2 [Google Scholar] [CrossRef]
[36]. Holstein N, Ginkgo biloba special extract EGb 761® in the treatment of tinnitusAn overview of the results of clinical trials. Fortschr Med Orig 2000 118(4):157-64. [Google Scholar]
[37]. Meyer B, Multicenter randomised double-blind drug vs. placebo study of the treatment of tinnitus with Ginkgo biloba extractPresse Médicale Paris Fr 1983 1986 15(31):1562-64. [Google Scholar]
[38]. Hahn A, Radkova L, Achiemere G, Klement V, Alpini D, Strouhal J, Multimodal therapy for chronic tinnitusInt Tinnitus J 2008 14(1):69-72. [Google Scholar]
[39]. Ribári O, Zelen B, Kollár B, Ethyl apovincaminate in the treatment of sensorineural impairment of hearingArzneimittelforschung 1976 26(10a):1977-80. [Google Scholar]
[40]. Gutmann R, Mees K, Piracetam-Infusionen bei akutem Tinnitus und Hörsturz Piracetam infusions in acute tinnitus and sudden deafnessFortschr Med 1995 113(18):288-90. [Google Scholar]
[41]. Ihl R, Bachinskaya N, Korczyn AD, Vakhapova V, Tribanek M, Hoerr R, GOTADAY Study GroupEfficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: A randomised controlled trialInt J Geriatr Psychiatry 2011 26(11):1186-94.10.1002/gps.266221140383 [Google Scholar] [CrossRef] [PubMed]
[42]. Doijad RC, Pathan AB, Pawar NB, Baraskar SS, Maske VD, Gaikwad SL, Therapeutic applications of citicoline and piracetam as fixed dose combinationAsian Journal of Biomedical and Pharmaceutical Sciences 2012 2(12):15-20. [Google Scholar]
[43]. Zhang W, Huang Y, Li Y, Tan L, Nao J, Hu H, Efficacy and safety of vinpocetine as part of treatment for acute cerebral infarction: A randomised, open-label, controlled, multicenter CAVIN (Chinese Assessment for Vinpocetine in Neurology) TrialClin Drug Investig 2016 36(9):697-704.10.1007/s40261-016-0415-x27283947 [Google Scholar] [CrossRef] [PubMed]