Pneumonia is an inflammatory response that occurs due to the uncontrolled replication of respiratory pathogens in the lungs [1]. CAP occurs due to alveolar infection that develops in the outpatient setting or within 48 hours of hospitalisation. CAP is of two types– typical and atypical [2].
Atypical pathogens have rarely been isolated from patients with CAP, because of the invasive nature of the effective methods or the requirement of explicit facilities for culture/serology of these organisms [4,5]. Atypical pathogens cannot be cultured on standard media or stained with gram stain neither do these respond to beta lactams [6]. Such atypical pathogens need to be detected to avoid coronary artery disease, multiple sclerosis, meningitis, meningoencephalitis, etc., [7].
Gramegna A et al., reported that the tests conducted and henceforth reported, to detect atypical pathogens is not adequate to conclude on its prevalence [8]. There is not much data available on aetiology of CAP from developing countries like India. Kumar KJ et al., studied pneumonia in children aged between 5 months to 2 years, wherein the atypical pathogens were found in 23 children (out of 38 with aetiological diagnosis) [9]. Similarly, a report from Srinagar showed Legionella pneumophila to be the second most common pathogen isolated from 17.5% of 225 patients [10].
For tailored therapy, knowledge of the potential pathogen is very important. Clinically, it is often difficult to predict the microbial aetiology on the basis of clinicoradiological picture. The Asian region being diverse, existing British and American guidelines cannot, rather should not be transported blindly to this region without some idea of local prevalence. To draft rational antibiotic guidelines studies should be done in different parts of the country to know the regional variations in CAP.
The present study was designed to establish the proportion of atypical respiratory pathogens and their clinical presentations in patients with CAP.
Materials and Methods
This was a prospective, cross-sectional study conducted in the Department of Microbiology, MS Ramaiah Medical College for a period of one year, spanning from January 2013 to February 2014. A total of 202 patients admitted in the Department of General Medicine and Chest Medicine in the study hospital were enrolled in the study. The study obtained permission from the Institutional Ethical Committee, vide letter number STD-1/EC/12-13.
Inclusion criteria: All patients aged 18 years and above with clinical and radiological features (non-homogenous opacity, lower zone consolidation, bilateral mid and lower zone opacity) compatible with CAP.
Exclusion criteria: Patients with ventilator associated pneumonia, hospital acquired pneumonia, previous hospital admission in the past one week, with radiological evidence of active tuberculosis, congestive cardiac failure, pulmonary infarction, lung cancer, patient who received more than two doses of antibiotics within the past 24 hours of sample collection, on immunosuppressive therapy and pregnant females.
Data collection: Patient history and demographic data, such as age, gender, date of admission, risk factors involved, underlying diseases, presenting complaints, antibiotic therapy, and other details were obtained. Clinical diagnosis of CAP and provisional diagnosis of atypical pneumonia were based on the British Thoracic Society [11] and the Japanese Respiratory Society Guidelines, respectively [12]. Blood samples were collected from all CAP patients as per the Joint Indian Chest Society (ICS) and National College of Chest Physicians (NCCP) (I) Recommendations for pneumonia and subjected to microbiological processing [6].
Indirect Immunofluorescence Assay (IFA): Approximately, 2 to 4 mL of whole venous blood was collected from all CAP patients. The samples were centrifuged at 1000 rpm for 10 minutes at 4°C. Serum was separated and stored at -20°C until Immunoglobulin M (IgM) levels were estimated, using the PNEUMOSLIDE-M IFA kit (Vircell, Granada, Spain) [7]. This test measured the levels of human serum IgM antibodies against the atypical CAP pathogens. Each slide had 10 wells, each containing one of the following antigens: L.pneumophila sero group 1, M.pneumoniae, C.burnetii, C.pneumoniae, adenovirus, RSV, influenza A, influenza B, parainfluenza serotypes 1, 2, 3 and cell control.
According to manufacturer’s instructions, serum samples were diluted 1:1 with Phosphate Buffered Saline (PBS) and then treated with anti-human IgG sorbent [13]. Sorbent treated diluted serum was incubated for 90 minutes at 37°C in the 10 well slides. After incubation, the slides were washed twice with PBS. A fluorescent secondary IgM antibody was added to the wells and incubated at 37°C for 30 minutes and then washed twice with PBS. A greenish-yellow coloured fluorescence indicated a positive IgM response.
Statistical Analysis
Descriptive analysis was done on the collected data which have been shown below in the form of mean and percentage.
Results
The study population constituted 128 (63.36%) males and 74 (36.64%) females. Of the 202 patients, 27 (13.37%) had typical pneumonia, 67 (33.17%) had atypical pneumonia, while in 108 (53.46%) patients no aetiological agents could be identified.
The patients were segregated into four age-groups: ≤20 years, 21-40 years, 41-60 years, and ≥61 years. Majority of the population concentrated in the age group of ≥61 years [Table/Fig-1].
Distribution of patients according to age group.
| Age group (years) | No. of patients (n=202) | Percentage (%) |
|---|
| ≤20 | 3 | 1.49 |
| 21-40 | 39 | 19.3 |
| 41-60 | 71 | 35.15 |
| ≥61 | 89 | 44.06 |
Patient Distribution Pattern in the Hospital
Out of the 202 patients, the majority of the patients were admitted to the ICU, accounting for 127 (62.87%) patients. This was followed by inpatient admissions, which accounted for 73 (36.14%) patients. The least number, accounting for just 2 (0.99%) patients, visited the Outpatient Department (OPD).
Common Pulmonary and Extrapulmonary Symptoms
The most common symptom, irrespective of patients with pulmonary or extrapulmonary infections was fever, which accounted for 198/202 (98.01%) of cases. Other symptoms, specifically pulmonary and extrapulmonary symptoms, are presented in [Table/Fig-2].
Major pulmonary and extrapulmonary symptoms.
| Symptoms | No. of patients (n=202) | Percentage (%) |
|---|
| Pulmonary |
| Dry cough | 135 | 66.83 |
| Chest pain | 94 | 46.53 |
| Breathlessness | 87 | 43.07 |
| Cough with expectoration | 43 | 21.29 |
| Extrapulmonary |
| Myalgia | 49 | 24.26 |
| Headache | 43 | 21.29 |
| Vomiting | 30 | 14.85 |
| Diarrhoea | 17 | 8.42 |
| Polyarthralgia | 9 | 4.46 |
| Others* | 19 | 9.41 |
*Migratory joint pain, pulmonary renal syndrome, meningo-encephalitis, cranial nerve palsy, conjunctivitis
Co-morbid Conditions
There were several co-morbid conditions (78 out of 202), which were primarily present in elderly patients. Two of the most common co-morbid conditions were Chronic Obstructive Pulmonary Disease (COPD) and Type-2 Diabetes Mellitus (T2DM) [Table/Fig-3].
Distribution of co-morbid conditions.
| Co-morbid conditions (78) | No. of patients | Percentage (%) |
|---|
| COPD | 20 | 25.6 |
| T2DM | 17 | 21.7 |
| Asthma | 14 | 17.9 |
| Chronic Kidney Disease (CKD) | 8 | 10.2 |
| Hypertension | 8 | 10.2 |
| Others* | 11 | 14.1 |
*RHD: Rheumatic heart disease; ARDS: Acute respiratory distress syndrome; myeloma, pleural effusion, non-Hodgkin’s lymphoma, hepatitis, multiple sclerosis; COPD: Chronic obstructive pulmonary disease; T2DM: Type 2 diabetes mellitus
Identification of Atypical Pathogens by Indirect IFA [
Table/Fig-4]
IFA positive atypical pathogens (a-i), and the cell contro (j); a) L. pneumophila; b) M. pneumoniae; c) C. pneumoniae; d) C. burnetii; e) Adenovirus; f) Respiratory Syncytial Virus (RSV); g) Influenza A; h) Influenza B; i) Parainfluenza 1,2,3; j) Cell control.
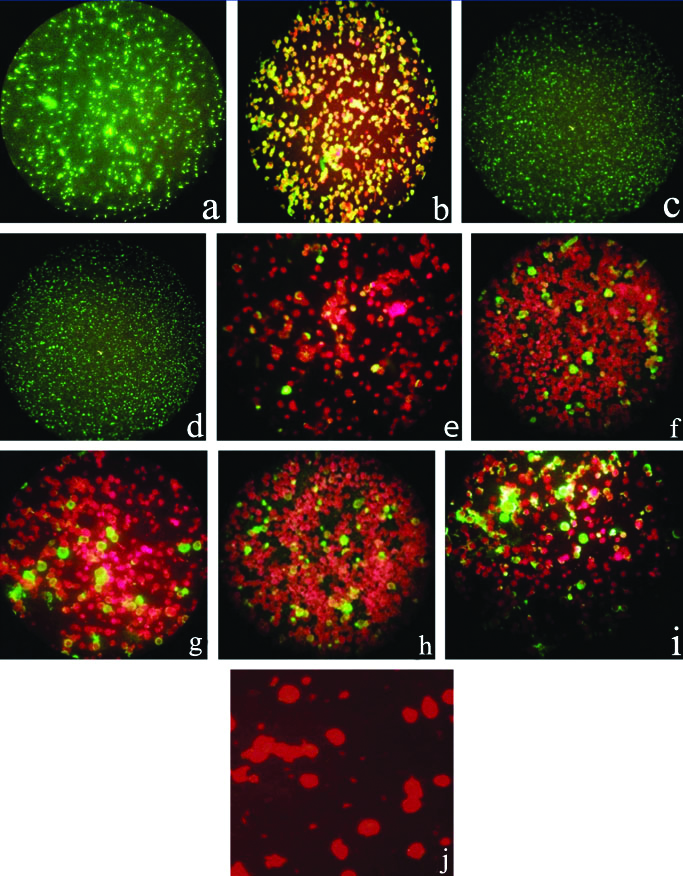
IFA Imaging
Imaging of atypical pathogens following IFA revealed the undermentioned patterns of staining, which are characteristic for the specific microorganism:
Apple green fluorescence in periphery of the cell: [Table/Fig-4b] M. pneumoniae
Apple green fluorescence in all the bacteria: [Table/Fig-4a] L. pneumophila, [Table/Fig-4c] C.burnetii, [Table/Fig-4d] C. pneumoniae
Apple green nuclear, cytoplasmic, and/or peripheral fluorescence in 1-15% of cells: [Table/Fig-4e] Adenovirus, [Table/Fig-4f] RSV, [Table/Fig-4g] Influenza A, [Table/Fig-4h] Influenza B, [Table/Fig-4i] Parainfluenza 1, 2, 3.
IgM Positivity
IFA for single atypical pathogen was found to be IgM-positive in 51 (25.24%) patients and for mixed pathogens, was found to be IgM-positive in 16 (7.92%) patients. Of these, mixed atypical infection was found to be IgM-positive in 14 (6.93%) patients and the remaining 2 (0.99%) patients had mixed infection with atypical and typical pathogens.
Proportion of Atypical Pathogens Responsible for Community Acquired Infections (CAP)
From [Table/Fig-5], it is clearly evident that the most common IgM response was found to be positive for M. pneumoniae (12.38%) among all CAP patients, followed by L. pneumophila (9.9%) and influenza A (5.94%).
Proportion of atypical pathogens in CAP.
| Atypical pathogens | No. of Atypical pathogens (Out of 202 patients) | Percentage (%) |
|---|
| M. pneumoniae | 25 | 12.38 |
| L. pneumophila | 20 | 9.9 |
| C. burnetii | 6 | 2.97 |
| C. pneumoniae | 4 | 1.98 |
| Adenovirus | 4 | 1.98 |
| Influenza A | 12 | 5.94 |
| Influenza B | 4 | 1.98 |
| RSV | 1 | 0.5 |
| Parainfluenza 1,2,3 | 7 | 3.47 |
| Total | 83 | 41.09 |
Mixed Infections
Concurrent infection caused by more than one pathogen is known as mixed infections. Mixed infections occurred in 16 patients, with the highest being L. pneumophila and Influenza A coinfection, which accounted for 4 patients. The data on mixed infections is presented in [Table/Fig-6].
Distribution of mixed infections among CAP patients.
| Pathogens causing mixed infections | No. of patients (n=202) |
|---|
| L. pneumophila + Influenza A | 4 (1.98%) |
| L. pneumophila + M. pneumoniae | 2 (0.99%) |
| M. pneumoniae + Adenovirus | 1 (0.50%) |
| Streptococcus pneumoniae + M. pneumoniae | 1 (0.50%) |
| C. pneumoniae + Influenza A | 1 (0.50%) |
| C. burnetii + Influenza A | 1 (0.50%) |
| C. burnetii + M. pneumoniae | 1 (0.50%) |
| L. pneumophila + Adenovirus | 1 (0.50%) |
| M. pneumoniae + Klebsiella pneumoniae | 1 (0.50%) |
| Influenza B + Parainfluenza 1,2,3 | 1 (0.50%) |
| M.pneumoniae + Parainfluenza 1,2,3 | 1 (0.50%) |
| M.pneumoniae + Adenovirus + Influenza A + Parainfluenza 1, 2,3 | 1 (0.50%) |
Age Predilection of Atypical Pathogens
L. pneumophila 9 (45%) and C. pneumoniae 4 (100%) were commonly seen in the elderly, age >61 years, whereas M. pneumoniae 9 (36%) was more common in individuals between 21-40 years of age. C. burnetii 6 (100%) was seen in the age group of 51-60 years.
Distribution of Atypical Pathogens in Different Hospital Wards
All 4 (100%) C. pneumoniae and 19 (76%) M. pneumoniae atypical CAP patients were admitted in the ICU. The most common atypical pathogen identified in the ICU and inpatient wards was M. pneumoniae (19vs6), followed by L. pneumophila (14vs5). One each, Coxiella and Influenza A atypical CAP patients had visited the OPD.
Discussion
In recent times, atypical respiratory bacteria, such as M. pneumoniae, L. pneumophila and C. pneumoniae are being increasingly isolated. Viruses such as influenza virus, adenovirus, and RSV, which are also important aetiologic agents of atypical pneumonia, are also being detected with increased frequency. Since clinical evaluation or X-rays are unable to accurately identify the aetiologic agent responsible for atypical pneumonia, microbiological and serological assays are required [4].
The proportion of atypical pathogens in the present study was 33.17%, which is similar to several other studies [Table/Fig-7] [5,7,14-18].
Proportion of atypical pathogens in CAP compared to other studies [5,7,14-18].
| Study | Publication year | Proportion of atypical pathogens (%) |
|---|
| Mundy LM et al., [7] | 1998 | 7.5% |
| Lieberman D et al., [5] | 1996 | 63% |
| Dey AB et al., [14] | 2000 | 35% |
| Ngeow YF et al., [15] | 2005 | 19.9% |
| Oberoi A and Aggarwal A [16] | 2006 | 34% |
| Zaki MES and Goda T [17] | 2009 | 60% |
| Agmy GM et al., [18] | 2010 | 29% |
| Current study | 2021 | 33.17% |
In the present study, atypical CAP was most commonly seen in the age group of >60 years. In a study by Ngeow YF et al., the common age group for acquiring atypical CAP was similar to the present study (51-60 years) [15]. The mean age of the patients in the present study was 57.18±17.09 years, while in case of another study it was >65 years [19].
In the present study, the most common co-morbid condition associated with atypical CAP was COPD (9.9%), followed by T2DM (8.42%). Nine out of 25 patients (36%), suffering from Mycoplasma pneumoniae had asthma, whereas 8 out of 20 Legionella pneumophila patients (40%) had COPD as the co-existing condition. In a study by Ngeow YF et al., T2DM was the most common co-morbid condition, followed by COPD [15].
In the present study, most of the patients had presented with complaints of fever and cough. In the study done by Abdullah BB et al., in elderly patients, cough was the most common respiratory symptom noted in 37 (74%) patients, which was productive in only 29 (58%) patients [20]. Other common symptoms included dyspnoea (22%), chest pain (20%), altered sensorium (16%), and gastrointestinal (GI) symptoms (8%).
IFA was found to be positive for 67 out of 202 (33.17%) CAP patients. In a similar study, Oberoi A and Aggarwal A found 34% of atypical respiratory pathogens among 232 CAP patients [16]. A study by Agmy GM et al., reported 29% of atypical pathogens by IFA [18]. Higher rates of atypical pathogens were seen in a study conducted at the All India Institute of Medical Sciences (AIIMS), New Delhi by Dey AB et al., where the prevalence of Mycoplasma in CAP was found to be as high as 35% [14]. To note is that, the AIIMS study included 12 immunocompromised participants, out of the 35%. In the present study, IgM response was most commonly found for M.pneumoniae (12.38%) followed by L.pneumophila (9.9%) and influenza A (5.94%).
The incidence of M.pneumoniae in hospitalised CAP patients usually varies from 0.8 to 29.2% [14]. In the present study, M.pneumoniae was identified in 12.38% of patients with CAP, which was similar to the results of a previous Asian study by Ngeow Y-F et al., (11.2%) [15]. Although pneumonia caused by M.pneumoniae is more frequent among children and young adults [21], the present results did not show any age predilection. However, it was more common in the age group of 21-40 years. On the other hand, a study on Vietnamese children showed an age predilection in terms of severity of the CAP caused by atypical pathogens. This large study was conducted on 722 hospitalised patients. Atypical pathogens were detected using multiplex PCR and ELISA. There were 215 atypical pathogen-positive CAP children. Among the 97 children with severe CAP, 54 were caused by pure atypical pathogens. M. pneumoniae was the most common aetiology found in 84 (out of 97) [22].
The incidence sporadic CAP caused by Legionella varies from 0.6 to 12.2% among cases requiring hospitalisation, depending on the geographic area and the diagnostic technique used [15]. In the present study, L.pneumophila sero group 1 was identified in 9.9% of CAP patients, which was similar (8%) to the findings of a previous study from Kuwait [4]. The present study also found that this pathogen was most commonly seen in patients over the age of 60 years.
C. pneumoniae is a frequent cause of CAP in hospitalised patients, with rates ranging from 3.4-43% [15,22] and is also associated with severe CAP [23-25]. In the present study, C. pneumoniae was incriminated for 2% of CAP cases and was responsible for 15% of severe pneumonia requiring ICU admission, which was slightly higher than that reported by some other studies [18,25]. All four cases of severe CAP caused by C. pneumoniae met the criteria of definitive diagnosis; two patients had prior chronic lung disease and two were previously healthy. Of the two previously healthy patients, one patient had coinfection with C. pneumoniae and influenza A. Among patients with C. pneumoniae pneumonia, underlying illness was absent in 56.6% of cases, and coinfection did not occur in 98% of cases. Therefore, it is felt that C. pneumoniae could be the sole cause of CAP requiring hospitalisation.
In this study, Coxiella burnetii was identified in 6 (2.97%) of patients with CAP, which is very similar to another study where C. burnetii found in 1-3% of pneumonia cases [26]. All these patients were in 41-60 years age group. Hepatitis was found in 4 (66.7%) out of 6 CAP patients with Coxiella burnetii infection in the present study.
In the present study, IgM response against influenza A was found in 12 (5.94%) CAP cases. Six out of 12 samples were sent for H1N1 Reverse Transcription Polymerase Chain Reaction (RT-PCR). But all were negative. Influenza B was positive in 4 (1.98%) cases. Also, IgM response was found to be positive for parainfluenza 1, 2, 3 in 7 (3.46%) cases. Of these, two patients had a history of bronchitis, while another was an elderly diabetic male. IgM response was positive against adenovirus and RSV in 1.98% and 0.5% of cases, respectively.
Mixed infections among CAP patients, especially coinfection by atypical bacterial pathogens is well-established [27]. In recently published studies, multiple pathogens were identified in 37% [28], 38% [23], and 48% [29] of all patients for whom an aetiologic agent was established. Mixed infections occurred in 16 patients in the present study. Among these, four patients had concurrent infections with L. pneumophila and influenza A and two patients had infection with M. pneumoniae and L. pneumophila. Importantly, it is often difficult to establish which pathogen in a mixed infection is the more important cause of disease. The study by Huong PLT et al., reported a total of 44.33% of their study population (children between 1-15 years of age) to be positive for mixed infection by typical pathogens and 55.67% with pure atypical ones [22].
Worldwide standard test methods for rapid detection of different atypical respiratory pathogens include Indirect IFA, Micro-Immunofluorescence (MIF), Enzyme Linked Immunosorbent Assay (ELISA), Complement Fixation Test (CFT) for serological diagnosis. PCR, antigen detection in urine (Legionella) and cell culture can also be used for definitive diagnosis of these pathogens [30].
Culture for viral and atypical bacterial isolation although sensitive but time consuming, takes almost two to three weeks. PCR technique is rapid, highly sensitive and specific but require specialised equipment, reagents and expertise [31]. According to the various literature it is concluded that, for most of the atypical pathogens, either single IgM response or four fold rise in antibody titre between acute and convalescent sera is diagnostic of infection [32]. For most of the cases, IFA is the recommended method for early detection of infections, provided the serum should be collected between 7 to 21 days of illness [33]. Arnold FW et al., conducted a study in four different regions (Region I: North America, Region II: Europe, Region III: Latin America Region IV: Asia and Africa) of the world and found that incidence of CAP due to atypical pathogens in the regions I to IV were 22, 28, 21, and 20%, respectively. The proportion of patients treated with atypical coverage were 91%, 74%, 53%, and 10% in regions I, II, III and IV, respectively [34]. They also studied to assess clinical outcomes of patients with CAP treated with and without atypical coverage, concluded that compared to those without atypical coverage, patients treated with atypical coverage had:
Decreased time to clinical stability (3.7 vs. 3.2 days)
Decreased length of stay (7.1 vs. 6.1 days)
Decreased total mortality (11.1% vs. 7%)
Decreased CAP related mortality (6.4% vs. 3.8%)
A secondary analysis of the Global Initiative for Meticillin-Resistant Staphylococcus aureus Pneumonia (GLIMP) database, showed that atypical pathogen for CAP testing frequency was highest in Europe. The analysis was on adult patients admitted for CAP in 222 hospitals across 6 continents in 2015. The study evaluated frequency of occurrence of L. pneumophila, M. pneumoniae, C. pneumoniae, and their prevalence. Among 3702 CAP patients 1250 (33.8%) underwent at least one test for atypical pathogens. Detection of L. pneumophila urinary antigen was the most common test performed. Additional findings of the study were that at least one atypical pathogen was isolated in 62 patients [8].
Overall, it can be stated that the presence of atypical pathogens have significant aetiological contribution to CAP. The present study reiterates the fact that these organisms should be studied individually and the inferences of such studies must be utilised in drafting treatment and management protocols for better therapeutic outcomes.
Limitation(s)
Combination of IgM and paired sera collected in acute and convalescent phase to demonstrate four fold rises in IgG antibody titre would have been better choice for definite diagnosis. As it was time consuming and reagent cost also matters so authors restricted to detect single IgM response against atypical pathogens. Nonetheless, most sensitive and specific method for definitive diagnosis is PCR which could not be performed due to costly reagents, invasive methods for sample collection and highly sophisticated instruments to run the same.
Conclusion(s)
This study indicates that there is a need for active screening for CAP cases in all wards and ICUs, since ICU admissions are on the rise. Since, differentiation between typical and atypical pneumonia is not possible based on clinical features alone, specific tests like IFA is required for rapid and accurate detection of the aetiologic agent. Accurate diagnosis will give an idea about the proportion of atypical CAP, which is vital for choosing the right antibiotic for a better prognosis.