Metabolic Syndrome (MetS) is an important public health burden affecting nearly a quarter of world’s population [1]. Factors involved with this syndrome include atherogenic dyslipidemias, central obesity and increased blood pressure and impaired fasting glucose levels. The syndrome is associated with fivefold risk of developing T2DM and two fold increase risk of developing CVD [2]. The major risk factors are diet [3], genetics [4], sedentary behaviour [5] or low physical activity, disrupted sleep [6], psychotropic medication [7] and excessive alcohol consumption [8].
According to NCEP ATP III criteria, the diagnosis of MetS is made when any three of the five following criteria are met: WC is 94 cm or higher in males and 80 cm or higher in females with any of the additional two criteria: increased triglyceride level (≥1.7 mmol/l or 150 mg/dL), low High Density Lipoprotein-Cholesterol (HDL-C) (≤1.0 mmol/l or 40 mg/dL) in males and (≤1.3 mmol/l or 50 mg/dL) in females, increased blood pressure (≥130 bpm systolic, ≥85 bpm diastolic) and impaired fasting glucose (≥5.6 mmol/l or 100 mg/dL).
In Asian Indians, the prevalence of MetS is 5% in rural population but increases to greater than one third in urban areas [9]. The incidence in India now ranges from 11% to 41% [10]. Asian Indians are metabolically obese but physiologically not obese [11]. MetS is a cluster of individual risk factors, drawing the attention of clinician to the probable co-existence of multiple cardio-metabolic risk factors in patients when one of the component is found [12]. For any given level of Body Mass Index (BMI), Asian Indians have increased prevalence of cardio-metabolic abnormalities as compared to other ethnic groups [13]. The goal is to target the different components of MetS with lifestyle and pharmacologic therapies to prevent disease particularly Cardiovascular Disease (CVD) and diabetes which is preceded by insulin resistance.
Bone regulates insulin-glucose axis and energy metabolism by secreting osteocalcin, an osteoblast specific protein [14]. Osteocalcin is a 49 aminoacid containing bone protein, implicated in MetS, is synthesised exclusively by the osteoblast and stored in bone mineral matrix. It becomes gamma carboxylated on three glutamic acid residues at positions 17, 21 and 24 forming bone Gamma-carboxy Glutamic Acid (GLA) protein. In the process of acidification of bone mineral matrix the carboxylated osteocalcin loses its glutamic acid residues and give rise to undercarboxylated osteocalcin, the hormonally active form that is released into the blood stream [15]. It stimulates proliferation of beta cells and insulin secretion through G-protein Coupled Receptor Class C Group 6 (GPRC6A) receptor which when activated, phosphorylates keyproteins of insulin secreting pathway in pancreatic beta cells. A decreased level of osteocalcin is found to be associated with development of MetS [16]. GPRC6A deletion is found in glucose intolerance and insulin resistance [17].
Osteocalcin especially the undercarboxylated form is found to be associated with glucose homeostasis. The aim of the current study was to investigate whether alteration in serum osteocalcin is associated with insulin resistance and the components of MetS and the change in the level of osteocalcin with increase in diagnostic criteria for MetS.
Materials and Methods
The present study was a hospital based case-control study which carried out in the Department of Biochemistry, MKCG Medical College, Brahmapur, Odisha, India. The study was carried out between December 2018 to January 2020. The study was conducted according to the ethical principle of medical research involving human subjects (Helsinki Declaration) and was approved by Institutional Ethical Committee bearing reference number of 672 of MKCG Medical College, Brahmapur, Odisha, India. Informed written consents were obtained from each individual prior to the study.
Sample size calculation: Sample size was calculated by using the formula: N=(Zα+Zβ)2 (SD)2/(Mean Difference)2
(Zα=The probability of falsely rejecting a true null hypothesis
Zβ=The probability of failing to reject a false null hypothesis)
and considering 95% confidence interval and 80% power of study to detect 1.1 ng/mL difference in the mean levels of osteocalcin among cases and controls using parameters from previous studies [18] the sample size was calculated to be 45.
Inclusion criteria
Cases: Forty eight individuals between the age group of 20-45 years meeting the NCEP ATP III criteria of MetS attending the Medicine OPD at MKCG Medical College and Hospital, Brahmapur, Odisha, India were taken as cases. Cases of MetS were again divided into three groups depending on the number of criteria present in each individual:
Group I- Cases with any of THREE criteria positive.
Group II- Cases with any of FOUR criteria positive.
Group III- Cases with all the FIVE criteria positive.
The NCEP ATP III criteria 2005 define MetS by presence of any three or more of the following criteria [19]:
Blood glucose ≥100 mg/dL,
High Density Lipoprotein (HDL) cholesterol ≤40 mg/dL in men, ≤50 mg/dL in women,
Blood triglycerides ≥150 mg/dL,
WC ≥102 cm in men or ≥88 cm in women and
Blood pressure ≥130/85 mmHg
Controls: Fifty individuals of age and sex matched healthy attendants of patients coming to the Outpatient Department of Medicine, staffs and students of MKCG Medical College without satisfying any of the criteria for MetS were chosen as controls for the study.
Exclusion criteria: Individuals with any kind of chronic illness or taking medications like oral hypoglycaemic drugs, insulin, statins, anti-hypertensive drugs, medications altering the vitamin K level, oral contraceptives, steroids etc., were excluded from the study. Hypertensives, alcoholics and smokers were also excluded as these can alter different biochemical parameters.
Study Procedure
Anthropometric parameters measurement: Height was measured using stadiometer, weight was measured using digital weighing machine, WC was measured using measuring tape placing the tape along the horizontal plane of the superior border of iliac crest and resting blood pressure was measured in supine position using sphygmomanometer. Body Mass Index (BMI) was calculated as weight (in kg)/{height (in mt)}2.
Routine parameters measurement: Fasting plasma glucose, serum total cholesterol, serum triglyceride, serum HDL, serum urea and creatinine were estimated using commercial kits by TOSHIBA120 FR autoanalyser and serum electrolytes were measured by i-Sense electrolyte analyser maintaining external and internal quality control at Regional Diagnostic Centre, MKCG Medical College and Hospital.
Special parameters measurement: Special parameters like serum osteocalcin and serum insulin were estimated using commercial kits by Enzyme Linked Immunosorbent Assay (ELISA) LISA SCAN READER and ROCHE e COBAS 411 electrochemiluminiscence respectively.
Parameters calculated: Ratios of TC/HDL and LDL/HDL calculated to assess the cardiovascular risk in MetS cases.
Insulin resistance HOMA-IR=
Insulin (μg/mL)×{glucose (mg/dL)×0.055/22.5}
The normal HOMA-IR value of healthy human ranges from 0.5-1.4.
Quantitative Insulin Sensitivity Check Index (QUICKI) for insulin sensitivity can be calculated by:
1/{log(Fasting plasma insulin μIU/mL)+log(Fasting plasma glucose mg/dL)}
Collection of sample for analysis: A sample of venous blood (7 mL) was collected in a dry, sterile disposable syringe under aseptic conditions after overnight fasting. A 2 mL of blood was kept in vial containing fluoride as glycolytic inhibitor and centrifuged for estimating fasting plasma glucose and fasting plasma insulin. A 2 mL of sample was kept in plain vial and was centrifuged for estimating other routine biochemical parameters. Rest of the sample was collected in plain vial and was centrifuged for estimating serum osteocalcin.
Storage of sample: The vials containing serum/plasma were labelled properly and were stored at -20°C. The samples can be stored upto a period of one month and can be thawed once. All the estimations were completed within 24 hours of collection of blood sample.
Statistical Analysis
The statistical analysis was done using SPSS version 22.0. Descriptive analysis was done to compare the baseline characteristics between cases and controls. Pearson Correlation of various components of MetS with serum osteocalcin was analysed and was represented graphically in form of scatter plots. Independent sample t-test was applied to estimate the special parameters between cases and controls. Association of various components of MetS with serum osteocalcin was found and p-value was also found. Analysis of Variance (ANOVA) test was done to compare the serum osteocalcin level with increase in criteria of MetS with calculated F value which is the ratio two mean square values. The p-value ≤0.05 was considered as statistically significant.
Results
A statistically significant difference in mean values of various biochemical parameters were observed between cases and controls. The mean±SD values of WC, BMI, systolic and diastolic blood pressure, fasting plasma glucose, serum total cholesterol, serum triglyceride were found to be statistically significantly higher in cases of MetS as compared to healthy controls [Table/Fig-1]. The mean±SD values of serum HDL was found to be higher in controls than in cases which was also statistically significant. The mean±SD values of lipoprotein ratios i.e. TC/HDL and LDL/HDL for assessing the cardiovascular risk factor was also found to be higher in cases than in controls indicating the risk of developing CVD was more in cases than in controls. The mean±SD values of special parameter i.e. serum osteocalcin was found to be higher in controls than in cases suggesting that serum osteocalcin may have protective role in preventing metabolic disorders. The serum fasting insulin and HOMA-IR values were found to be statistically significantly higher in cases as compared to controls suggesting that MetS cases are insulin resistant. The insulin sensitivity measured as Quantitative Insulin Sensitivity Check Index (QUICKI) score was found to statistically significantly higher in healthy controls than in MetS cases.
Comparison of mean of various parameters between cases and controls using independent sample t-test.
| Parametres | Cases(Mean±SD) (N=48) | Controls(Mean±SD)(N=50) | t-value | p-value |
|---|
| Age (years) | 36.40±7.04 | 34.67±6.70 | 8.65 | 0.656 |
| BMI (Kg/m2) | 33.28±3.81 | 24.08±3.59 | 12.35 | <0.001* |
| WC (cm) | 113.60±13.60 | 87.98±10.03 | 10.64 | <0.001* |
| SBP (mmHg) | 149.25±19.87 | 123.40±5.92 | 6.81 | <0.001* |
| DBP (mmHg) | 92.50±10.00 | 79.00±5.80 | 9.98 | <0.001* |
| FPG (mg/dL) | 122.40±38.71 | 89.74±8.95 | 5.81 | <0.001* |
| TC (mg/dL) | 212.10±65.54 | 158.58±28.28 | 5.28 | <0.001* |
| TG (mg/dL) | 214.00±149.84 | 121.15±48.26 | 4.16 | <0.001* |
| HDL (mg/dL) | 31.58±13.65 | 47.07±10.50 | -2.91 | <0.001* |
| Urea (mg/dL) | 21.37±8.70 | 19.15±3.72 | 1.580 | 0.118 |
| Creatinine (mg/dL) | 0.77±0.25 | 0.80±0.28 | -0.454 | 0.651 |
| TC/HDL | 6.88±4.48 | 4.39±2.16 | 6.75 | <0.001* |
| LDL/HDL | 5.89±5.81 | 1.78±0.97 | 5.76 | <0.001* |
| Sr. Osteocalcin (ng/dL) | 7.74±4.62 | 23.24±9.74 | -9.96 | <0.001* |
| Sr. Insulin (μIU/mL) | 20.63±8.20 | 8.68±3.81 | 9.36 | <0.001* |
| HOMA-IR | 6.31±3.28 | 1.87±0.76 | 9.30 | <0.001* |
| Quantitative Insulin Sensitivity Check Index (QUICKI) | 0.30±0.24 | 0.37±0.04 | 8.32 | <0.001* |
*p-value <0.05 was statistically significant; BMI: Body mass index; WC: Waist circumference; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; TC: Total cholesterol; TG: Tiglyceride; TC/HDL: Total cholesterol/High density lipoprotein; LDL/HDL: Low density lipoprotein/High density lipoprotein; Sr: Serum; HOMA-IR: Homeostatic model assessment for insulin resistance
[Table/Fig-2] shows Pearson correlation between various parameters of MetS with serum osteocalcin. It was found that the serum osteocalcin was negatively correlated with all the parameters except for serum HDL and QUICKI score for insulin sensitivity which are positively correlated with serum osteocalcin in both cases and controls.
Pearson correlation of various parameters with serum osteocalcin.
| Parameters | Cases | Controls |
|---|
| r | p-value | r | p-value |
|---|
| WC (cm) | -0.519 | <0.001* | -0.176 | 0.245 |
| SBP (mmHg) | -0.480 | <0.001* | -0.225 | 0.153 |
| DBP (mmHg) | -0.492 | <0.001* | -0.121 | 0.444 |
| FPG (mg/dL) | -0.539 | <0.001* | -0.141 | <0.05 |
| TG (mg/dL) | -0.401 | 0.005* | -0.398 | <0.05 |
| HDL (mg/dL) | 0.758 | <0.001* | 0.193 | 0.221 |
| TC/HDL | -0.500 | <0.001* | -0.384 | 0.012 |
| LDL/HDL | -0.550 | <0.001* | -0.445 | 0.003 |
| INSULIN (μIU/mL) | -0.133 | 0.368 | -0.378 | 0.014 |
| HOMA-IR | -0.322 | 0.025* | -0.494 | <0.001 |
| QUICKI | 0.242 | 0.097 | 0.362 | 0.023 |
*p-value <0.05 is significant; QUICKI: Quantitative insulin sensitivity check index
[Table/Fig-3] describes that with increase in number of criteria for MetS, there is decrease in serum osteocalcin level. It also describes that there is increase in insulin resistance and decrease in insulin sensitivity with increase in criteria for MetS.
Comparison of mean of various parameters with increase in number of criteria of Metabolic Syndrome (MetS).
| Parameter | Metabolic syndrome with three criteria positive | Metabolic syndrome with four criteria positive | Metabolic syndrome with five criteria positive |
|---|
| Mean±SD | Mean±SD | Mean±SD |
|---|
| Serum Osteocalcin (ng/dL) | 13.18±2.99 | 7.30±1.43 | 3.01±1.58 |
| HOMA-IR | 3.77±0.91 | 5.74±1.22 | 8.19±3.99 |
| QUICKI | 0.308±0.01 | 0.305±0.01 | 0.291±0.03 |
[Table/Fig-4] ANOVA test was applied for comparison of mean serum osteocalcin with increase in severity of MetS and it was found that with increase in severity of MetS there is decrease in serum osteocalcin level.
ANOVA test for comparison of mean of serum osteocalcin with increasing severity of Metabolic Syndrome (MetS)
| Parameter | Sr. Osteocalcin(Mean±SD) | Test statistics |
|---|
| MS with 3 criteria | 13.18±2.99 | F=93.547,p<0.01 |
| MS with 4 criteria | 7.3±1.43 |
| MS with 5 criteria | 3.33±1.59 |
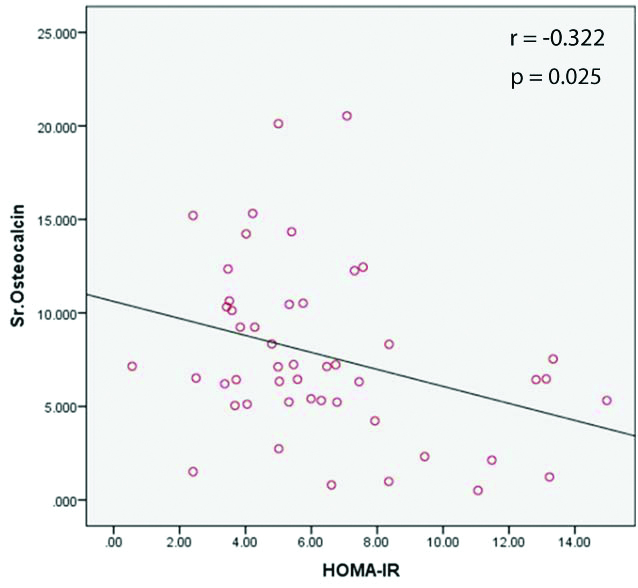
[Table/Fig-5] shows scatter plot showing negative correlation between serum osteocalcin and insulin resistance.
Correlation of serum osteocalcin with HOMA-IR.
Discussion
In present study, it was found that serum osteocalcin is lower in MetS cases compared to healthy controls. WC, which is considered an important criterion for development of MetS was found to be significantly higher in cases with a strong negative correlation with serum osteocalcin. WC is one of the measures for Visceral Fat Amount (VFA) and is associated with insulin resistance and development of MetS. Kanazawa I et al., reported that serum osteocalcin levels were negatively correlated with trunk fat and VFA in male subjects with T2DM [20]. Also, Bao Y et al., study found an inverse association between serum osteocalcin levels and VFA in Chinese men after adjusting for potentially confounding factors [21].
In the present study higher values of both Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) were found in cases as it is one of the important criterions for MetS. But, a negative correlation was obtained between osteocalcin and both SBP and DBP which was statistically significant. Several works already documented negative association between total and carboxylated osteocalcin with SBP and DBP which suggest a protective role of osteocalcin on blood pressure [22]. On the other hand, a positive correlation was found between the above two parameters in the study conducted by Sanchez-Enriquez S et al., [23].
In the present study, serum osteocalcin was found to be negatively correlated with serum triglyceride level and positively correlated with serum HDL level which was similar to the findings of Kindblom JM et al., TC/HDL Ratio and LDL/HDL ratio were both found to be statistically higher in the MetS group compared to controls with a strong negative association with the osteocalcin level [24]. Sanchez-Enriquez S et al., found a negative correlation of undercarboxylated osteocalcin with HDL-c and a positive correlation with TC/HDL-c and LDL-c/HDL-c ratios in diabetics. Also, an inverse relationship was found between osteocalcin and atherosclerotic parameters in diabetic males [20]. These studies point out to the role of osteocalcin in regulation of lipid metabolism and its possible cardio-protective effects.
Previous studies revealed a significant negative association of serum osteocalcin level with fasting plasma glucose level [25]. In present study, though the cases on antidiabetic medications were excluded and thus have included only the newly diagnosed diabetics and impaired fasting plasma glucose tolerant cases, but nonetheless got a strong negative association between FPG and serum osteocalcin. This can be explained by the facts that osteocalcin stimulates pancreatic beta cells to release insulin, which acts on the Insulin Receptors expressed on the osteoblasts and inhibits the osteoprotegerin that stimulate the osteoclastic activity. The osteoclastic activity results in acidification of extracellular matrix which causes decarboxylation of osteocalcin resulting in formation of the active undercarboxylated form that is released into the bloodstream. Patti A et al., study demonstrated that the deletion of IR in the osteoblasts lead to an impaired release of osteocalcin from the bone with negative effect on glucose tolerance [25]. Pi M et al., [17], study suggested that osteocalcin activates GPRC6A receptor which activates the IP3-Ca+2 pathway by the action of phospholipase C that yields the secretion of insulin. In the pancreas binding of osteocalcin to GPRC6A induces Erk (extracellular signal regulated kinases) phosphorylation and increases insulin synthesis.
In the present study, a significantly higher Fasting Insulin (FINS) value was found with increase in insulin resistance in MetS cases compared to controls which may point towards a state of hyperinsulinemia that occurs as a manifestation of insulin resistance before established T2DM can present. Though the interrelationship between FINS and osteocalcin was not significant, there was a statistically significant negative association between osteocalcin and insulin resistance calculated as HOMA-IR which is corroborated with the findings of other authors [26]. Insulin resistance occurs due to overabundance of circulating free fatty acids. Insulin is an anti-lipolytic hormone, thus when there is insulin resistance there occurs increased lipolysis of triacylglycerol producing more free fatty acids. Increased free fatty acids causes increased intra-mitochondrial acetyl CoA and increased NADH/NAD+ ratio with subsequent inactivation of pyruvate dehydrogenase. There is an increase in citrate concentration with inhibition of phosphofructokinase. There occurs subsequent accumulation of Glucose-6-Phosphate with inhibition of hexokinase-II activity resulting in decreased intracellular glucose uptake [27]. A decrease in the insulin sensitivity was also found by QUICKI score, though not statistically significant. No significant difference was found with serum osteocalcin, HOMA-IR and QUICKI values between the sexes, though serum osteocalcin level was marginally higher in females.
In present study it was found that with increase in degree of severity of MetS, as evidenced by three, four or all five criteria, there is a decrease in level of serum osteocalcin and insulin sensitivity with simultaneous increase in insulin resistance. Mean osteocalcin was higher in people having MetS satisfying 3 criteria as compared to those who satisfy 4 criteria. Serum osteocalcin is least in people with MetS satisfying 5 criteria. Confavreux CB et al., study also found a strong negative association of serum osteocalcin level with increase in severity of MetS [28].
Limitation(s)
In future estimation of serum adiponectin level in both cases and controls and its correlation with serum osteocalcin, serum fasting insulin, insulin resistance (HOMA-IR) and insulin sensitivity can be included in the study.
Conclusion(s)
The findings of present study suggest an important role of osteocalcin in the regulation of insulin secretion, sensitivity and resistance. Also, the strong association of different cardiovascular risk factors and lipid markers in particular with osteocalcin points towards its role in regulation of lipid metabolism. The possible therapeutic role of osteocalcin in the prevention of insulin resistance and CVD cannot be ruled out and needs active research.
*p-value <0.05 was statistically significant; BMI: Body mass index; WC: Waist circumference; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; TC: Total cholesterol; TG: Tiglyceride; TC/HDL: Total cholesterol/High density lipoprotein; LDL/HDL: Low density lipoprotein/High density lipoprotein; Sr: Serum; HOMA-IR: Homeostatic model assessment for insulin resistance
*p-value <0.05 is significant; QUICKI: Quantitative insulin sensitivity check index