Vanishing Non-immune Hydrops in Giant Chorioangioma of Placenta
Sunita Dubey1, Aayushi Kaushal2, HN Pavithra3
1 Assistant Professor, Department of Obstetrics and Gynaecology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
2 Senior Resident, Department of Obstetrics and Gynaecology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
3 Demonstrator, Department of Pathology, Government Medical College and Hospital, Sector 32, Chandigarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Aayushi Kaushal, JK Hospital, Palm City, Attewali, Near Mata Sundri School, Fatehgarh Sahib, District Fatehgarh Sahib, Punjab, India.
E-mail: kaushalaayushi@gmail.com
Giant Chorioangioma of placenta is a rare nontrophoblastic tumour of placenta. It may lead to various maternal and foetal complications like massive antepartum haemorrhage, sudden intrauterine foetal demise and non-immune hydrops, although in few cases mother and the foetus remain unaffected. This report is of a 35-year-old G3P1L1A1, presented to hospital at 32 weeks gestation with pain abdomen followed by watery discharge from vagina. Ultrasonography at 30 weeks revealed a huge mass on anterior wall with placenta on posterior wall of uterus although her previous antenatal sonography did not reveal any abnormality either in the foetus or in placenta. Diagnosis of preterm rupture of membranes was confirmed. Hence, she was kept on conservative management; received antibiotics and steroids for foetal lung maturity. Subsequently, the foetus developed mild, steady non-immune hydrops probably due to high output cardiac failure as Values of Middle Cerebral Artery’s Peak Systolic Velocity (MCA-PSV) were within normal limits. Biophysical profile and nonstress test were normal. Guarded foetal prognosis was given due to non-immune hydrops but she delivered a normal female baby with good Appearance, Pulse, Grimace, Activity and Respiration (APGAR) score with huge chorioangioma of placenta. Although rare, chorioangiomas of placenta should be kept in differential diagnosis of non-immune hydrops that needs regular foetal surveillance and timely intervention in affected foetuses to increase survival after birth.
Nontrophoblastic tumour of placenta, Polyhydramnios, Preterm labour, Preterm rupture of membranes
Case Report
A 35-year-old G3P1L1A1 at 32+3 weeks gestation referred to the hospital with complaint of labour pains. She had one normal vaginal delivery 17 years back, followed by spontaneous abortion one year later. Antenatal period was uneventful till admission. Her family and medical history were not significant.
Ultrasonography of the foetus at 18 and 28 weeks gestation did not reveal any abnormal mass within the placenta or uterine wall and there was no evidence of any malformations in the foetus too. Her vitals were stable, fundal height was of 32 weeks gestation. Ultrasound revealed foetal parameters of 30 weeks with fundoposterior placenta and a well-defined multilobulated, hypoechoic mass measuring 98×75 mm with hyperechoic bands, arising from the chorionic plate of anterior uterine wall was protruding inside the amniotic cavity [Table/Fig-1].
Transabdominal Ultrasonography revealed placenta (long arrow) with separate hypoechoic multilobulated mass (small arrow) with minimal blood flow on colour doppler study.
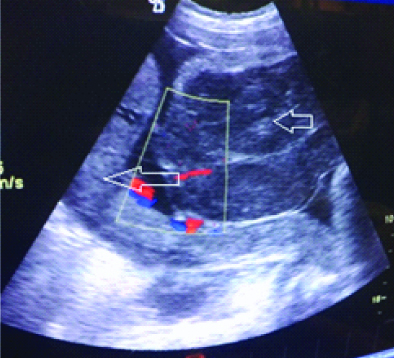
She received four doses of 6 mg dexamethasone given by intramuscular route at six hourly intervals for foetal lung maturity and was discharged following cessation of uterine contractions with advise of follow-up in Outpatient Department. After one week, she presented with complaints of watery discharge per vaginum that was confirmed by vaginal examination. Ultrasound revealed; live foetus with breech presentation, reduced liquor (Amniotic Fluid Index: 3 cm) along with the evidence of mild foetal scalp oedema and pericardial effusion. Thin streak of fluid was seen around the left kidney suggestive of ascites. Abdominal wall muscles and overlying skin appeared thick and hypoechoic suggestive of subcutaneous oedema with similar placental mass as noticed earlier. Patient was admitted for evaluation of foetal hydrops and management of preterm rupture of membranes along with antibiotic cover as per our hospital protocol and monitored for signs and symptoms of chorioamnionitis.
First abruptio placenta was excluded as she did not have any episode of bleeding from vagina and there was no evidence of increase in fundal height and uterine tenderness on clinical examination. Differential diagnosis of succenturiate lobe and submucosal fibroid uterus were made initially. After development of mild foetal ascites and pericardial effusion, these diagnosis were revised and diagnoses of placental tumour was made as a cause of hydrops due to difference in echotexture of mass and pattern of blood flow.
To rule out structural defects and arrhythmias as a cardiac cause of foetal hydrops, foetal echocardiography was done that revealed mild pericardial effusion and mild cardiomegaly. Foetal anaemia was also excluded by Middle Cerebral Artery-Peak Systolic Velocity (MCA-PSV) doppler study, value of which was 1.0 Multiple of Median (MoM) for gestational age (40 cm/s at 32 weeks). Viral infections were excluded by negative Toxoplasma, Rubella, Cytomegalovirus, Herpes Simplex Virus (TORCH) profile. Maternal thalassaemia screening and Indirect Coomb test were also done to exclude inherited cause of foetal anaemia and possibility for alloantibodies other than anti-Rh D, respectively. Possibility of aneuploidy was also excluded as echocardiography and level II scan did not reveal any structural abnormality.
Subsequent monitoring by ultrasonography revealed normal liquor and features of hydrops were steady. MCA-PSV colour Doppler study was within normal limits with good biophysical profile.
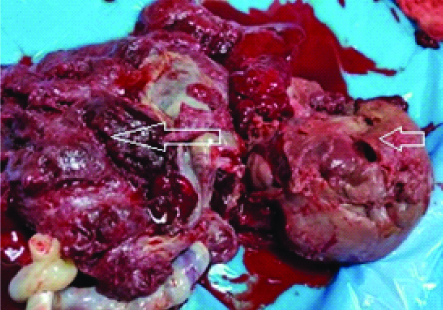
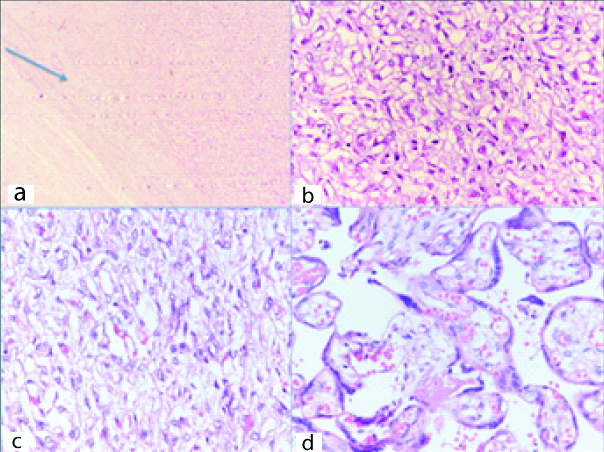
Guarded foetal prognosis was explained to the relatives but they opted for caesarean section. At 34 weeks she delivered a live born girl of 1.7 kg birth weight with APGAR score of 9, 9 at 1 and 5 minute, respectively. Following delivery of the placenta, a 10×8.5×6.2 cm fleshy vascularised mass was removed, histological examination confirmed angiomatoid type of chorioangioma [Table/Fig-2,3].
Gross specimen of placenta (long arrow) with solid mass (small arrow) suggestive of Placental tumour.
H&E stain; 3a: 4X objective- Circumscribed lesion demarcated from the surrounding; 3b: 20x and 3c 40x objective- Proliferation of capillary sized blood vessels lined by plump endothelial cells; 3d: 40x objective- Proliferation of blood vessels causing expansion of villi.
On imaging by ultrasonography and chest X-ray, there was no evidence of hydropes found in the baby. Her haemoglobin was 16 gm%, sepsis screen was negative. Baby is currently two-year-old with adequate psychomotor development and growth.
Discussion
Chorioangioma of placenta is a rare but most frequent among nontrophoblastic benign tumour of placenta which arise from the vessels of chorionic tissue. This tumour has three different histological types like angiomatous, cellular and degenerative type; out of them angiomatous type is most commonly reported [1]. Most of them are small in size (<4 cm), with an incidence of 1 in 100 pregnancies that is usually asymptomatic but may contribute to raised alpha fetoprotein only [2]. Whereas giant chorioangioma (>4 cm) has an incidence of 1 in 500 to 1 in 16000 pregnancies which may leads to various maternal and foetal complications [3,4].
Due to high perinatal mortality (30-40%) there is a need of timely interventions either in utero or in neonatal period particularly in cases of foetal hydrops [5]. Very few case reports of giant chorioangioma of placenta with intrauterine non-immune hydrops have resulted in good neonatal outcome [6-9]. However, present case of mild foetal hydrops was also born with good outcome without any intervention that otherwise would have required in massive foetal hydrops, merits it’s reporting.
Polyhydramnios, preterm labour are the most common maternal complications, whereas maternal mirror syndrome and near miss mortality have also been noticed in patient with giant chorioangioma of placenta [10-12]. In the present case, her amniotic fluid levels were normal even after preterm rupture of membranes which might be contributed to chorioangioma of placenta. So far, neonatal outcome has been reported favourable in the few cases only and minority of them may have foetal growth restriction only [4]. Conversely, spontaneous fetomaternal haemorrhage may lead to sudden intrauterine deaths and foetal anaemia [13].
Foetal hydrops is a serious consequence of chorioangioma of placenta that is defined as presence of fluid collection in any two of the following cavities (abdominal, pericardial and pleural cavity or skin oedema >5 mm). In placental chorioangioma, foetal hydrops may be caused either by anaemia following sequestration of foetal RBC within the tumour or due to increased cardiac fluid overload, as assumed in present case. However, on doppler study; blood flow within the tumour was not so profuse that may be the reason why foetus continued to have mild foetal hydrops. Some authors have reported partial or complete resolution of hydrops in neonatal period similar to present case, reason being necrosis within the placental tumour leading to decreased blood flow within the tumour [8,13,14].
Disseminated intravascular coagulation-like picture have been observed in neonates, secondary to formation of microthrombi within the tumour [14]. Higher incidence of infantile haemangioma and neonatal mortality due to purpura fulminance has been reported with chorioangioma of placenta [15]. Thrombosis of umbillical vein, cerebral infarction and cerebral ischemic stroke are various complications reported in the neonatal period [16,17]. Malignant potential in chorioangioma has also been reported with no evidence of maternal and foetal metastasis [18]. Therefore, continuous surveillance of baby is required even in neonatal period to detect chorioangioma related complications.
Indomethacin can be used in polyhydramniose besides amnioreduction to avoid preterm labour [19]. However, severe constriction of the ductus arteriosus or tricuspid regurgitation needs discontinuation of this drug [20]. Intrauterine foetal transfusion can be used as a supportive treatment for foetal anaemia whereas laser ablation of the tumour is the definitive treatment for foetal anaemia as well as polyhydramniose [21]. Non-availability of laser ablation warrants ultrasound guided alcohol injection and microcoil or enbucrilate embolisation within or nearest to the tumour. Hence, for these procedures patients need to be referred to higher centre. Whereas, unaffected foetus needs regular surveillance with ultrasound only.
Conclusion(s)
Prognosis of foetal hydops is usually guarded but in mild foetal hydrops of placental etiology can be unexpectedly good especially in third trimester with normal foetal MCA-PSV Doppler study. Coagulation of the feeding vessels by intrauterine endoscopic laser can be done to improve outcome in affected foetuses.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Nov 17, 2020
Manual Googling: Jan 09, 2021
iThenticate Software: Jan 23, 2021 (5%)
[1]. Marchetti AA, A consideration of certain types of benign tumours of the placentaSurg Gynecol Obstet 1939 68:733-43. [Google Scholar]
[2]. Amer HZ, Heller DS, Chorangioma and related vascular lesions of the placenta-a reviewFetal Pediatr Pathol 2010 29:199-206.10.3109/15513815.2010.48700920594143 [Google Scholar] [CrossRef] [PubMed]
[3]. Zanardini C, Papageorghiou A, Bhide A, Thilaganathan B, Giant placental chorioangioma: Natural history and pregnancy outcomeUltrasound Obstet Gynecol 2010 35:332-36.10.1002/uog.745119859897 [Google Scholar] [CrossRef] [PubMed]
[4]. Wu Z, Hu W, Clinical analysis of 26 patients with histologically proven placental chorioangiomasEur J Obstet Gynecol Reprod Biol 2016 199:156-63.10.1016/j.ejogrb.2015.12.00926927893 [Google Scholar] [CrossRef] [PubMed]
[5]. Batukan C, Holzgreve W, Danzer E, Bruder E, Hösli I, Tercanli S, Large placental chorioangioma as a cause of sudden intrauterine fetal death. A case reportFetal Diagn Ther 2001 16(6):394-97.10.1159/00005394611694744 [Google Scholar] [CrossRef] [PubMed]
[6]. Caldas RT, Peixoto AB, Paschoini MC, Adad SJ, Souza ML, Araujo Júnior E, Giant placental chorioangioma with favorable outcome: A case report and literature review of literatureCeska Gynekol 2015 80(2):140-43. [Google Scholar]
[7]. Fan M, Mootabar H, A rare giant placental chorioangioma with favorable outcome: A case report and review of the literatureJ Clin Ultrasound 2015 43(4):254-56.10.1002/jcu.2218725043806 [Google Scholar] [CrossRef] [PubMed]
[8]. Chazotte C, Girz B, Koenigsberg M, Cohen WR, Spontaneous infarction of placental chorioangioma and associated regression of hydrops fetalisAm J Obstet Gynecol 1990 163(4 Pt 1):1180-81.10.1016/0002-9378(90)90684-Y [Google Scholar] [CrossRef]
[9]. Asokan S, Chad AK, Gard R, Prenatal diagnosis of placental tumour by ultrasoundJournal of Clinical Ultrasound 1978 6:180-81.10.1002/jcu.187006031497323 [Google Scholar] [CrossRef] [PubMed]
[10]. Yadav M, Maheshwari M, Sharma S, Godha Z, Garg P, Sharma G, Chorioangioma of placenta: A rare case of near-miss mortalityJ Obstet Gynaecol India 2017 67(3):224-26.10.1007/s13224-016-0934-728546672 [Google Scholar] [CrossRef] [PubMed]
[11]. García-Díaz L, Carreto P, Costa-Pereira S, Antiñolo G, Prenatal management and perinatal outcome in giant placental chorioangioma complicated with hydrops fetalis, fetal anemia and maternal mirror syndromeBMC Pregnancy Childbirth 2012 28:12-72.10.1186/1471-2393-12-7222840187 [Google Scholar] [CrossRef] [PubMed]
[12]. Kawano R, Takemoto S, Shimamatsu K, Hori D, Kamura T, Fetomaternal haemorrhage with intraplacental chorioangiomaJ Obstet Gynaecol Res 2013 39(2):583-87.10.1111/j.1447-0756.2012.01996.x22925543 [Google Scholar] [CrossRef] [PubMed]
[13]. Willis C, Ferguson S, Soydemir F, Placental chorioangioma associated with polyhydramnios and hydrops fetalisBMJ Case Rep 2019 29(12)(1)10.1136/bcr-2018-22782830700468 [Google Scholar] [CrossRef] [PubMed]
[14]. Abiramalatha T, Sherba B, Joseph R, Thomas N, Unusual complications of placental chorioangioma: Consumption coagulopathy and hypertension in a preterm newbornBMJ Case Rep 2016 2016:bcr201621573410.1136/bcr-2016-21573427154993 [Google Scholar] [CrossRef] [PubMed]
[15]. Selmin A, Foltran F, Chiarelli S, Ciullo R, Gregori D, An epidemiological study investigating the relationship between chorangioma and infantile hemangiomaPathol Res Pract 2014 210(9):548-53.10.1016/j.prp.2014.04.00724836731 [Google Scholar] [CrossRef] [PubMed]
[16]. Sivasli E, Tekşam O, Haliloğlu M, Güçer S, Orhan D, Gürgey A, Tekinalp G, Hydrops fetalis associated with chorioangioma and thrombosis of umbilical veinTurk J Pediatr 2009 51(5):515-18. [Google Scholar]
[17]. Ghidini A, Locatelli A, Diffuse placental chorioangiomatosis causing multiple fetal cerebral embolism: a case reportJ Reprod Med 2006 51(4):321-24. [Google Scholar]
[18]. Ariel I, Boldes R, Weintraub A, Reinus C, Beller U, Arbel R, Chorangiocarcinoma: A case report and review of the literatureInt J Gynecol Pathol 2009 28(3):267-71.10.1097/PGP.0b013e31818f127f19620945 [Google Scholar] [CrossRef] [PubMed]
[19]. Moise KJ Jr, Indomethacin therapy in the treatment of symptomatic polyhydramniosClin Obstet Gynecol 1991 34(2):310-18.10.1097/00003081-199106000-000121868638 [Google Scholar] [CrossRef] [PubMed]
[20]. Kriplani A, Abbi M, Banerjee N, Roy KK, Takkar D, Indomethacin therapy in the treatment of polyhydramnios due to placental chorioangiomaJ Obstet Gynaecol Res 2001 27(5):245-48.10.1111/j.1447-0756.2001.tb01264.x11776505 [Google Scholar] [CrossRef] [PubMed]
[21]. Hosseinzade P, Shamshirsaz AA, Javadian P, Espinoza J, Gandhi M, Ruano R, Prenatal therapy of large placental chorioangiomas: Case report and review of the literatureAJP Rep 2015 5(2):e196-e202.10.1055/s-0035-155882926495184 [Google Scholar] [CrossRef] [PubMed]