Supraclavicular brachial plexus blockade is one of the most commonly performed anaesthetic techniques used to provide anaesthesia for upper limb surgeries. Peripheral nerve blockades provide excellent surgical anaesthesia as well as postoperative analgesia without major systemic side-effects and also minimises stress response eventually leading to minimal systemic analgesic drug usage postoperatively [1]. The ease of performing the block at the level of the trunk where the plexus is most compact makes this approach an excellent choice not only allowing smaller volumes of local anaesthetic drug producing reliably intense blockade but also provides optimal tourniquet coverage. Ropivacaine is a longer acting alternative that is structurally related to bupivacaine with reduced potential for toxicity and improved sensory and motor blocking profiles [2].
To tackle the drawback of shorter duration of postoperative analgesia, an extensive array of adjuvants have been tried along with local anaesthetics for brachial blockade in upper limb surgeries and has shown significantly promising results in clinical practice [3-7].
Nalbuphine, a derivative of 14-hydroxymorphine, is an agonist-antagonist opioid acting on μ (mu) receptors as antagonist and κ (kappa) receptors as agonist with an analgesic potency equal to morphine and its antagonistic potency is approximately one fourth of that of naloxone [8]. Unlike morphine, it exhibits a ceiling effect on respiratory depression conferring superiority in expanding its clinical application [9]. Nalbuphine has the onset of action between 2 and 3 minutes, duration of action of 3-6 hours with cardiovascular stability, and minimal side-effects in the dose range of 0.2-0.4 mg/kg [10].
In view of its recent popularity and safety profile, Nalbuphine has frequently been used to treat burns pain in children besides its use in neoplastic and haematological diseases [11]. The other major advantage of nalbuphine use in day care surgeries is its exemption from controlled drug act regulations along with its superior analgesic profile making it an unmatched agent of choice for the purpose [12,13]. Nalbuphine has also acquired a significant place in pain control as a better analgesic than other opioids for postoperative pain relief in short surgical procedures but its efficacy as a local anaesthetic adjuvant is yet to be proved in peripheral nerve blockades [14-16].
The current study was designated to evaluate the efficacy of adding nalbuphine to ropivacaine in supraclavicular brachial plexus blockade and assess the quality of block for patients undergoing ambulatory forearm and hand surgery with the primary outcomes being duration of analgesia, onset of sensory and motor blockade, duration of motor blockade and secondary outcomes being haemodynamic changes during the procedure respectively.
Materials and Methods
This randomised controlled study was conducted in Department of Anaesthesiology, during the period of May 2019 to December 2019. The study was approved by the Institutional Ethics Committee (IEC:RC/19/52), prior to the commencement and written informed consent from patients was obtained. Clinical Trials Registry India (CTRI) number (CTRI/2020/06/025889).
Inclusion and Exclusion criteria: Seventy ASA grade I and II patients between age group of 18 to 60 years, undergoing elective forearm and hand surgeries were included. Patients with infection at the site of injection, clinically significant coagulopathy, pre-existing neuromuscular disorders, severe cardiovascular or pulmonary diseases, renal or hepatic disorders, pregnancy and lactation and those taking opioids or chronic analgesic therapy for any other illnesses were excluded from the study.
Sample size calculation: Taking into consideration the duration of analgesia as the main outcome of interest and from the previous study in which the mean value of duration of analgesia being 531±41.23 minutes in the study group and 501±42.12 minutes in the control group (difference of 30 mins) with a power of study of 80% and a significance level of 5%, the sample size was derived to be 30 in each group. Considering allowance for 10% dropouts, the sample size was increased to 35 in each group [17].
Patients were allocated into two groups, 35 patients in each group using computer generated randomisation table. Group A (n=35): Received 24 mL of 0.5% of ropivacaine + 1 mL of nalbuphine (10mg) and Group B (n=35): Received 24 mL of 0.5 % of ropivacaine + 1 mL of normal saline. Anaesthesiologist involved in the data collection as well as the patients were blinded to the content of the study solution as it was prepared by an anaesthesiologist not involved in the study.
All patients were premedicated with oral Lorazepam 1 mg, oral Ranitidine 150 mg HS and 2 hours prior to the approximate time of surgery with sips of water. After shifting the patient to operating table, intravenous access was secured with an 18 G cannula on the contralateral upper limb. Electrocardiography (ECG), Non-invasive Blood Pressure (NIBP), pulse oximetry monitor according to ASA standards was connected and baseline values were recorded and O2 at 4L/min was administered with simple face mask. Supraclavicular block was performed by a trained anaesthesiologist under aseptic precautions with the patient in supine position, head turned to the opposite side with shoulder support and hand to be blocked in an adducted position and completely extended to reach the ipsilateral knee as much as achievable. Under ultrasound guidance, brachial plexus was identified with the help of Sonosite M Turbo high frequency probe (6-13 Mhz linear array) through supraclavicular approach. Once brachial plexus was identified, a skin wheal with 2% lignocaine was raised and the block performed by using 20 gauge Braun needle. The location of the needle tip was confirmed by hydro-dissection with 0.9% normal saline and study drug was deposited inside the brachial plexus sheath after negative aspirations for blood or air visualising adequate spread.
Sensory blockade was assessed by checking for pain by pin prick test using the blunt end of a 27 gauge needle in the dermatomal areas corresponding to median nerve, radial nerve, ulnar nerve and musculocutaneous nerve and was compared with the contralateral upper limb till complete sensory blockade was achieved. Onset of sensory block was considered when there was a dull sensation to pin prick along the distribution of any of the above mentioned nerves. Complete sensory block was considered when there was a complete loss of sensation to pin prick. Sensory block was graded as-Grade 0: Sharp pain felt, Grade 1: Analgesia, dull sensation felt, Grade 2: Anaesthesia, no sensation felt [18].
Motor blockade was graded as Grade 0: Normal flexion and extension of elbow, wrist, and fingers, Grade 1: Decreased motor strength with ability to move the fingers only, Grade 2: Complete motor block with inability to move the fingers [19]. Onset of motor blockade was considered when there was Grade 1 motor blockade and complete motor block was considered when there was Grade 2 motor blockade [20]. Both sensory and motor blockade were assessed at 0 minute, and every 5 minutes for the first 30 minutes.
The block was considered incomplete when sensory anaesthesia was not achieved even after a systemic supplemental opioid (Inj. Fentanyl 1 mcg/kg) bolus within 30 minutes of drug injection and more than one nerve remained unaffected or when the local infiltration was not adequate. In this case the block was considered as failed and general anaesthesia was instituted and the patient was excluded from the study [20].
All patients were monitored for haemodynamic variables such as heart rate, blood pressure and oxygen saturation immediately and after the block for every 5 minutes for the first 15 minutes and then every 15 minutes then on till the end of the surgery. The duration of motor blockade was assessed taking into account the time taken from the onset of motor block till the patient regained the motor activity, like return of finger movements.
The duration of analgesia was assessed considering the time taken from the onset of sensory blockade till the request for first rescue analgesic dosage i.e., Visual Analog Scale (VAS) score >3. Injection diclofenac sodium 75 mg was given intramuscularly as rescue analgesia. Any complications like haematoma, block failure, pneumothorax, neuropraxia, bradycardia, local anaesthetic toxicity; sedation if any intraoperatively and during the postoperative period for 24 hours was also noted.
Statistical Analysis
All data were collected and entered in Microsoft Excel and analysed using standard statistical software Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were analysed using the Pearson’s Chi-square test. Continuous variables were analysed using the independent sample t-test and p<0.05 was considered as statistically significant.
Results
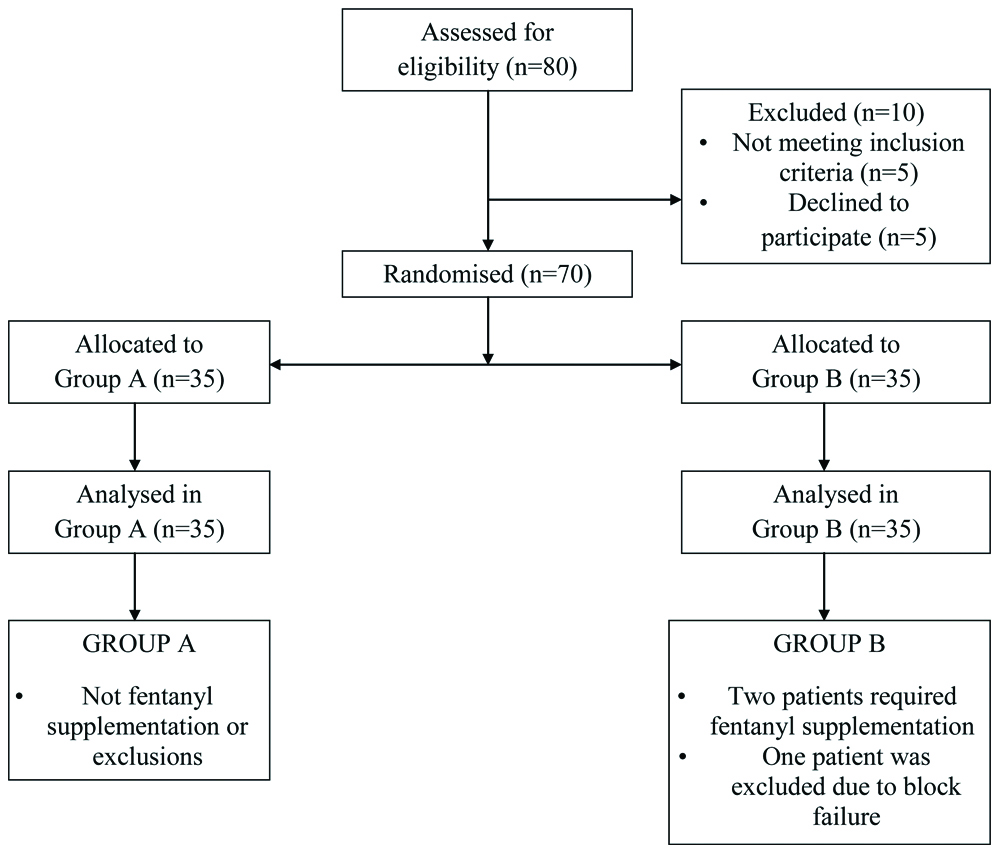
Seventy patients were enrolled for the study and were randomly divided into two groups. A consort flow outlining the method of recruitment and analysis is depicted in [Table/Fig-1]. The demographic profiles of patients in both the groups were comparable with regards to age, sex distribution and weight as denoted in [Table/Fig-2].
Demographic profile of patients in both group.
| Variables | Group A (35) | Group B (34) | Chi-square test p-value |
|---|
| Age in years (mean±SD) | 35.74±11.12 | 33.63±9.65 | 0.399 |
| Sex ratio (M:F) | 27:8 | 25:9 | |
| Weight in (kg) | 66.91±12.25 | 65.54±12.07 | 0.639 |
| ASA ratio (I:II) | 28:7 | 24:10 | |
The mean time for onset of sensory block was 5.20±2.87 min in Group A and 8.34±3.81 minutes in Group B. The mean time for onset of motor block was 8.51±3.81 minutes in Group A and 11.31±5.73 minutes in Group B. The statistical analysis by student’s unpaired t-test showed that there was a significant difference in the mean onset times of sensory and motor block between the two groups with p-value <0.001 which was statistically significant [Table/Fig-3].
Onset of sensory and motor blockade.
| Onset of block | Group A | Group B | Students unpaired t-test p-value |
|---|
| Onset of sensory block (min) | 5.20±2.87 | 8.34±3.81 | <0.001 |
| Onset of motor block (min) | 8.51±3.81 | 11.31±5.73 | 0.016 |
p-value <0.05 considered significant
The mean duration of sensory analgesia was 17±2.30 hours in group A and 12.93±3.34 hours in group B. The mean duration of motor blockade was 12.92±2.45 hours in group A and 10.85±2.49 hrs in group B. The statistical analysis by student’s unpaired t-test showed that there was a significant difference in the mean duration of sensory and motor block between the two groups with p-value <0.001 which was also statistically significant [Table/Fig-4].
Duration of sensory and motor blockade (hours).
| Duration | Group A | Group B | Students unpaired t-test p-value |
|---|
| Duration of sensory block (h) | 17±2.30 | 12.93±3.34 | 0.001 |
| Duration of motor block (h) | 12.92±2.45 | 10.85±2.49 | <0.001 |
p-value <0.05 considered significant
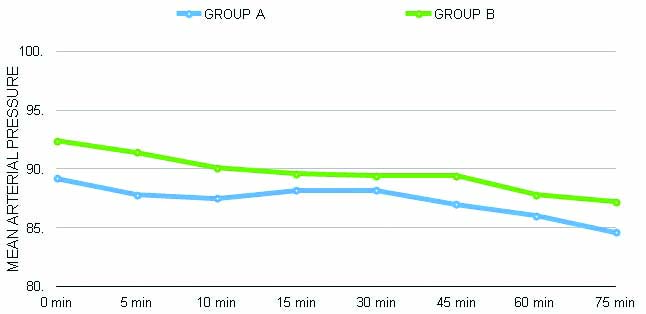
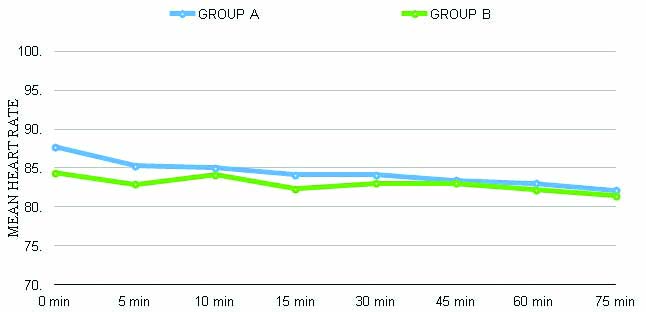
There were no significant haemodynamic changes observed between both groups, as depicted in [Table/Fig-5,6]. None of the patients required fentanyl supplementation in the nalbuphine group intraoperatively, whereas two patients required supplementation in the control group. One patient had a block failure in the saline group and was excluded from the study.
Mean arterial pressure changes (p>0.05).
Heart rate change (p>0.05).
Discussion
The designated end points of the current study were to evaluate the efficacy of adding nalbuphine to ropivacaine in supraclavicular brachial plexus blockade and assess the quality of block for patients undergoing ambulatory forearm and hand surgeries. The observations from this study clearly showed that nalbuphine has greater supremacy over any other adjuvant added to brachial plexus blockade.
The onset of sensory and motor blockade was significantly shorter in Group A which received 24 mL of 0.5% of ropivacaine+1 mL of nalbuphine (10 mg) compared to Group B which received 24 mL of 0.5% of ropivacaine+1 mL of normal saline. This could probably be attributed to the collective action of both nalbuphine and ropivacaine on accentuating the sensory blockade. The earlier onset of sensory blockade exhibited by ropivacaine has been well documented by other studies too that compared ropivacaine with bupivacaine in supraclavicular blockade [20,21]. However, the results of this study were comparable with Nazir N and Jain S who evaluated the efficacy of adding nalbuphine to bupivacaine in supraclavicular block. They inferred that addition of nalbuphine as an adjuvant, not only significantly reduced the onset and peak times of both sensory and motor blockade but also significantly prolonged the duration of analgesia in the study population [22].
The duration of sensory and motor blockade was also significantly prolonged in Group A against Group B. Similar findings were observed by Gupta K et al., [23]. They added nalbuphine as an adjuvant to 0.5% bupivacaine in USG guided Supraclavicular Brachial Plexus (SCB) blockade. They observed that nalbuphine significantly enhanced the duration of sensory and motor blockade and postoperative analgesia (481.53±42.45 min) in the study group compared to control group (341.31±21.42 min) with no adverse effects of nalbuphine usage; nevertheless no difference in onset times were observed between the groups [23]. Another similar inference was observed by Abdelhaq MM and Elramely MA while studying the effects of adding 1 mL of 10 mg nalbuphine to 0.5% bupivacaine to normal saline, on 56 subjects randomised into two groups. Their study showed that nalbuphine group had a significant increase in the duration of motor block (412.59±18.63 min), when compared to control group (353.70±29.019 min) with p-value <0.001. Sensory duration was in-turn significantly prolonged in the nalbuphine group (718.14±21.04 min) when compared to control group (610.18±26.33 min), without affecting the onset times of the blockade. There was a significant increase in the duration of analgesic effect in nalbuphine group (835.18±42.45 min) when compared to control group (708.14±54.57 min) [24].
The other consideration about unaffected onset times in both the above stated studies, even with addition of nalbuphine, was attributed to the bupivacaine use when compared to ropivacaine used in this study along with nalbuphine, the combination of which eventually culminated in significantly shorter onset times. This finding is in line with a recent study done by Jain K et al., which demonstrated shorter onset times with nalbuphine and ropivacaine combination in supraclavicular blockade though the difference was not statistically significant but definitely had a clinical value [25]. Das A et al., recently did a study evaluating the addition of nalbuphine as an adjuvant to levobupivacaine in USG guided supraclavicular blockade, enrolling 78 patients into two groups to receive either levobupivcaine and nalbuphine or normal saline. They observed that the duration of sensory and motor blockades were increased in the nalbuphine group and was both clinically and statistically significant [17].
No other drug or block related complications were encountered in either of the groups, in the index study, similar to previous studies [17,19,20,25].
Limitation(s)
Major limitations of this study could be the dose standardisation of nalbuphine to 10 mg due to non-availability of proper pharmaceutical reference relating to dose equivalence with other well-known opioids.
Conclusion(s)
Nalbuphine when added to ropivacaine for USG guided supraclavicular brachial plexus blockade significantly shortens the onset and prolongs the duration of both sensory and motor blockade with increased duration of postoperative analgesia without any adverse effects and is a safer adjuvant when used in upper limb blockade.
p-value <0.05 considered significant
p-value <0.05 considered significant