Introduction
The occurrence and progression of diabetic nephropathy to end stage disease mandates an early detection of kidney damage. Glycation and oxidation injury form an essential element in the evolution of diabetic complications both microvascular and macrovascular.
Aim
To study the trends in the N-Carboxymethyl lysine levels in various stages of diabetic nephropathy and assess its efficacy as a prognostic marker for diabetic nephropathy.
Materials and Methods
The study included 125 Type 2 diabetic individuals- 45 patients with normoalbuminuria, 40 patients having microalbuminuria and 40 with macroalbuminuria {based on the Albumin-Creatinine Ratio (ACR)}. Forty five non-diabetic healthy individuals were included as a control group. Serum N-carboxymethyl lysine quantification was done for all the study participants and compared and correlated with other parameters across various groups.
Results
The fasting and postprandial sugar, glycosylated haemoglobin, triglycerides, duration of diabetes, systolic and diastolic blood pressure, Body Mass Index (BMI), all were strong risk factors for Diabetic Kidney Disease (DKD) progression which significantly correlated positively with microalbumin and urine ACR (uACR) and negatively with Glomerular Filtration Rate (GFR). The serum N-carboxymethyl lysine was observed to be significantly increased as the ACR increased and in comparison to the controls, respectively (p-value <0.001). The GFR showed significant negative correlation with levels of serum N-carboxymethyl lysine whereas positively correlated with fasting and postprandial sugar, glycosylated haemoglobin, triglyceride levels, duration of diabetes, systolic and diastolic blood pressure, BMI, microalbumin and uACR.
Conclusion
N-carboxymethyl lysine in serum can serve as an early marker for diabetic nephropathy and its progression and severity.
Introduction
Diabetic nephropathy is the leading cause of renal failure and a common complication among the diabetic patients. Despite the usage of reno-protective therapies, the diabetic patients are prone to develop end stage renal disease [1].
Moreover, identifying the risk population for an early evaluation of DKD with the currently available biomarkers is difficult. The traditionally accepted concept held that microalbuminuria heralds macroalbuminuria which in due course leads to gradual decline in GFR leading to end stage renal disease in DKD [1-4]. Few systematic longitudinal studies gave refinements to this traditional concept. Microalbuminuria is a dynamic process which can revert to normal albumin excretion and some individuals can have early linear decline of GFR well ahead of macroalbuminuria onset and structural lesions may be present within the kidney even in the absence of clinical indicators of disease, which are available currently [5-7]. This emphasises the need for early prognostic non-invasive biomarkers of diabetic nephropathy that can identify new onset of microalbuminuria and estimated GFR (eGFR) decline well before the onset of irreversible structural changes.
The diabetic complications are mainly caused by persistent hyperglycaemia and tight glycaemic control slows the development and progression of both the microvascular complications and the structural lesions associated with diabetic nephropathy [8-10]. Hyperglycaemia causes diabetic complications partly through the non-enzymatic protein glycation and production of Advanced Glycation End products (AGEs) and its related Oxidative End Products (OPs) [11]. Both oxidation and glycation play significant roles in the genesis of these adducts. Non-enzymatic glycation of proteins (amino acids) by the reducing sugars and dicarbonyl compounds produce AGEs. The glycoxidation adducts thus formed are more chemically reactive and irreversibly cross-link proteins. Diabetic patients have been found to have higher concentrations of AGEs and glycoxidation adducts compared to healthy individuals. They are considered to increase the oxidative stress by interaction with their Receptor for Advanced Glycation Endproducts (RAGE) and further promote microvascular complications [12-15].
AGEs are filtered through the glomerulus and reabsorbed in the proximal tubules of the kidneys. Their reabsorption in tubules and clearance are variable [16]. With the accumulation of AGE in the glomeruli, there is an increased type IV collagen and laminin expression in extracellular matrix leading to irreversible cross-linking of proteins and premature cell senescence in the proximal tubules [17,18]. AGEs serve as signal transducing ligands for the transmembrane receptor RAGE, which by reactive oxygen species production and pro-inflammatory and pro-fibrotic cascade induces cellular injury [19]. Thus, aim of the study was to determine the prognostic role of serum N-Carboxymethyl lysine, one of the AGE products in the DKD.
Materials and Methods
A prospective case-control study was conducted over a period of 24 months from January 2018 to December 2019, in a Medical College Hospital in Southern India. The study was approved by the Institutional Ethics Committee with the reference number GSLMC/RC:395-EC/395-12/2016 and IEC-NI/16/NOV/56/75. The patients were enrolled in the study after obtaining an informed written consent.
The study enrolled 170 individuals with 125 patients having diabetes and 45 healthy controls. Among the diabetic patients, 45 had normoalbuminuria, 40 had microalbuminuria and 40 were macroalbuminuric.
Inclusion criteria: Patients diagnosed with Type 2 Diabetes Mellitus (T2DM) as per the World Health Organisation (WHO) criteria with fasting blood glucose ≥126 mg/dL or oral glucose tolerance test 2 hour prandial glucose ≥200 mg/dL and above 18 years of age.
Exclusion criteria: Patients with systemic hypertension on treatment with drugs which can interfere with proteinuria like angiotensin converting enzyme inhibitor or angiotensin receptor blockers were excluded. In addition to this, patients with urinary tract infections, any acute febrile illness, cardiovascular diseases and malignancies were also excluded as these conditions can confound the assessment of proteinuria.
A detailed history, clinical characteristics, duration of the disease, their baseline investigations, glycaemic control of the patients were documented in a pre-structured proforma and correlated across various stages of nephropathy. The diabetic patients were grouped based on their uACR. Based on the Joint Committee of Diabetic Nephropathy Classification [20] the diabetic patients were grouped into normoalbuminuria, microalbuminuria and macroalbuminuria when their uACR was less than 30 mg/g, 30-299 mg/g and more than or equal to 300 mg/g, respectively.
The carboxymethyl lysine level was assessed using Enzyme Linked Immunosorbent Assay (ELISA) method and compared between the study groups and with other study parameters between cases and controls.
Statistical Analysis
The data was analysed using Statistical Package for the Social Sciences (SPSS) software version 18.0. The quantitative data was represented as mean and Standard Deviation (SD). Comparison between more than two groups of normally distributed quantitative variables was done by using the Analysis of Variance (ANOVA) (F test) followed by post-hoc analysis and to compare mean between two groups, the Student’s t-test was applied. Sensitivity and specificity were used to assess the diagnostic performance of carboxymethyl lysine and to assess the overall performance Area Under Curve (AUC) was used.
Results
A total of 125 patients with 45 normoalbuminuria, 40 microalbuminuria and 40 macroalbuminuria and 45 healthy controls were included. The cases had 65.88% males and 34.12% females with mean age (SD) of 51.82 (11.94) years. The carboxymethyl lysine levels across the various study groups were 1238.96±124.45 ng/L, 1713±145 ng/L, 2041.72±176.38 ng/L in the normoalbuminuric, microalbuminuric and the macroalbuminuric diabetic patients, respectively and 1138.44±82 ng/L in the control group. [Table/Fig-1,2] show the descriptive and comparative statistics of the clinical and laboratory parameters among the various study groups.
Demographic and clinico-laboratory characteristics of study population.
| Characteristics | Cases | Controls (n=45) | p-value in between groups | p-value in comparison to control | Overall p-value |
|---|
| Normoalbuminuric (n=45) | Microalbuminuric (n=40) | Macroalbuminuric (n=40) | P1 | P2 | P3 | P4 | P5 | P6 |
|---|
| Age (years) Mean±SD | 50.95±12 | 52.83±12 | 53.71±10.2 | 49.58±9.66 | 0.159 | 0.039 | 0.521 | 0.284 | 0.015 | 0.002 | 0.009 |
| Male | 29 | 28 | 25 | 30 | - | - | - | - | - | - | - |
| Female | 16 | 12 | 15 | 15 |
| BMI (kg/m2) Mean±SD | 27.29±4.7 | 27.73±3.8 | 31.31±4.65 | 24.73±1.5 | 0.369 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Duration of DM (years) | 8.13±2.86 | 8.85±3.24 | 8.8±3.37 | ----------- | 0.376 | 0.441 | 0.913 | - | - | - | <0.001 |
| Systolic BP (mmHg) | 140.2±4.7 | 147.4±5.8 | 152±8.12 | 136.2±6.58 | <0.001 | <0.001 | 0.021 | 0.027 | <0.001 | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 86.58±6.2 | 92.74±5.4 | 96.42±4.27 | 82.46±3.16 | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | <0.001 |
| FBS (mg/dL) | 133.60±31 | 123.87±21 | 157.27±21 | 75.71±9 | 0.048 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| PPBS (mg/dL) | 195.86±46 | 181.37±30 | 222.12±37 | 113.64±13 | 0.052 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HbA1c (%) | 6.99±1.85 | 7.03±1.96 | 8.45±1.54 | 5.16±0.6 | <0.001 | <0.001 | <0.001 | 0.0795 | <0.001 | <0.001 | <0.001 |
| Urea (mg/dL) | 33.44±10.5 | 28.03±7.48 | 45.32±13.5 | 37.57±7 | 0.005 | <0.001 | <0.001 | <0.010 | <0.001 | <0.001 | <0.001 |
| Creatinine (mg/dL) | 0.89±0.24 | 0.82±0.18 | 2.185±0.54 | 0.78±0.2 | 0.004 | <0.001 | <0.001 | <0.002 | <0.001 | <0.001 | <0.001 |
| GFR (mL/min/1.73 m2) | 86.62±27.5 | 75.6±21.48 | 31.32±12.3 | 107.56±18 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Cholesterol (mg/dL) | 183.91±34 | 175.10±35.20 | 202±28.4 | 175.96±24 | 0.190 | 0.008 | <0.001 | 0.233 | 0.899 | <0.001 | <0.001 |
| Triglyceride (mg/dL) | 170.16±56 | 180.30±63 | 197.63±46 | 134.64±19 | 0.341 | 0.011 | 0.115 | <0.001 | <0.001 | <0.001 | <0.001 |
| LDL (mg/dL) | 109.15±29.03 | 100.22±31.79 | 125.15±25 | 107.25±24 | 0.095 | 0.012 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Urine albumin creatinine ratio | 25.46±3.25 | 84.9±4.30 | 351.43±35 | 25.07±3.27 | <0.001 | <0.001 | <0.001 | 0.947 | <0.001 | <0.001 | <0.001 |
| Microalbumin (mg/dL) | 24.88±3.23 | 84.7±35.7 | 296±41.9 | 25.07±3.27 | <0.001 | <0.001 | <0.001 | 0.973 | <0.001 | <0.001 | <0.001 |
| Serum AGE (ng/L) | 1238.95±124 | 1713±145 | 2041.72±176 | 1138.44±82 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 |
(BMI: Body mass index; FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; GFR: Glomerular filtration rate; LDL: Low density lipoprotein) (P1-between normoalbuminuric group and microalbuminuric group; P2-between normoalbuminuric group and macroalbuminuric group; P3-between microalbuminuric group and macroalbuminuric group, P4-between control group and normalbuminuric group; P5-between control group and microalbuminuric group; P6-between control group and macroalbuminuric group; Statistical analysis were done using student t-test and intergroup comparisons -ANOVA and post-hoc analysis)
Correlation between serum CML (carboxy-methyl lysine) levels and different parameters in each group.
| Characteristics | Serum CML (Carboxy-methyl lysine) (ng/L) | p-value (case-control) |
|---|
| Cases | Controls |
|---|
| n | Mean±SD | p-value | n | Mean±SD | p-value |
|---|
| Age (years) | <40 | ---- | --- | 0.081 | ----- | --------- | 0.831 | 0.008 |
| 41-50 | 49 | 1584.95±351.61 | 25 | 1144.92±89.45 |
| 51-60 | 62 | 1659.45±385.38 | 20 | 1138.44±80.86 |
| >60 | 14 | 1813.78±283.26 | ------- | ------- |
| BMI (kg/m2) | <23 | 3 | 1497.33±262.8 | <0.001 | 4 | 1152.75±87.70 | 0.139 | <0.001 |
| 23-25 | 14 | 1498.92±289.17 | 27 | 1123.63±77.39 |
| >25 | 108 | 1670.97±373.96 | 14 | 1162.92±88.91 |
| Duration of DM (years) | <5.0 | - | ------ | 0.326 | ---- | ------ | ---- | ---- |
| 5-10 | 105 | 1652.28±369.14 | ---- | ------ |
| >10 | 20 | 1622.60±358.23 | ----- | ------ |
| Systolic BP (mmHg) | <120 | - | ---- | <0.001 | ----- | -------- | 0.332 | <0.001 |
| 121-140 | 32 | 1410.28±321.26 | 37 | 1137.7±80.13 |
| >140 | 93 | 1729.17±345.85 | 8 | 1139.88±90.65 |
| Diastolic BP (mmHg) | <80 | 5 | 1197.6±177.68 | <0.001 | 11 | 1134.8±87.32 | 0.043 | <0.001 |
| 80-90 | 53 | 1463.90±315.75 | 34 | 1139.61±81.70 |
| >90 | 67 | 1826.37±310.71 | ----- | ------- |
| FBS (mg/dL) | <110 | 22 | 1479.18±321.54 | <0.001 | 45 | 1138.44±82.12 | 0.605 | 0.001 |
| 110-126 | 26 | 1523.92±247.65 | - | ------- |
| >126 | 77 | 1737.37±385.22 | - | ------- |
| PPBS (mg/dL) | <140 | 2 | 1251.50±19.09 | 0.002 | 45 | 1138.44±82.12 | 0.311 | <0.001 |
| 140-200 | 72 | 1583.93±331.04 | - |
| >200 | 51 | 1749.61±391.34 | - |
| HbA1C (%) | <7.0 | 8 | 1477.64±279.69 | <0.001 | 45 | 1138.44±82.12 | 0.444 | <0.001 |
| ≥7.0 | 117 | 1753.44±375.20 | - |
| BUN (mg/dL) | <30 | 123 | 1640.79±365.20 | <0.001 | 45 | 1144.07±82.65 | 0.054 | 0.052 |
| ≥30 | 2 | 2062±65.05 | - | 1093.4±68.74 |
| S.Triglycerides (mg/dL) | <150 | 38 | 1548.60±38.15 | 0.231 | 41 | 1138.44±82.12 | 0.810 | <0.001 |
| ≥150 | 87 | 1690.74±371.35 | 4 | 1138.44±82.12 |
| LDL (mg/dL) | <100 | 53 | 1578.83±334 | 0.263 | 33 | 1126.87±80 | 0.020 | <0.001 |
| ≥100 | 72 | 1698.11±382 | 12 | 1170.25±81 |
| S.Creatinine (mg/dL) | <1.2 | 81 | 1451.09±273.32 | <0.001 | 45 | 1138.44±82.12 | 0.030 | <0.001 |
| ≥1.2 | 44 | 2009.15±202.83 |
| uACR (mg/G) | <30 | 45 | 1238.95±124.44 | <0.001 | 45 | 1138.44±82.12 | 0.112 | <0.001 |
| 30-300 | 40 | 1713±145.99 | - | - |
| >300 | 40 | 2041.72±176.38 | - | - |
| eGFR (mL/min/1.73 m2) | ≥90 | 23 | 1370.91±285.17 | <0.001 | 45 | 1138.44±82.12 | 0.201 | <0.001 |
| 60-89 | 58 | 1490.5±263.91 | - | - |
| 45-59 | 5 | 1653.4±297.20 | - | - |
| 30-44 | 23 | 2077.30±154.85 | - | - |
| 15-29 | 16 | 1994.81±202.79 | - | - |
| <15 | - | - | - | - |
FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; GFR: Glomerular filtration rate; BUN: Blood urea nitrogen; LDL: Low density lipoprotein; uACR: Urinary albumin creatinine ratio; eGFR: Estimated glomerular filtration rate (student t-test and ANOVA were used to assess the statistical significance)
[Table/Fig-3] correlates the N-carboxymethyl lysine with various parameters in case and control groups. One hundred and five patients had diabetes for 5-10 years with 20 patients having diabetes for more than 10 years, but none with less than five years duration. A statistically significant difference in the serum N-carboxymethyl lysine levels between the disease groups as well as in comparison to the control group (p<0.001) was observed, however it did not correlate with the duration of diabetes.
Correlation between serum CML (Carboxy-Methyl Lysine) (ng/L) and different parameters in each group.
| Serum CML (Carboxy-methyl lysine) (ng/L) |
|---|
| Parameters | Cases | Control |
|---|
| r-value | p-value | r-value | p-value |
|---|
| Age (years) | 0.157 | 0.081 | -0.033 | 0.831 |
| BMI (Kg/m2) | 0.438 | <0.001 | 0.224 | 0.139 |
| Duration of DM (years) | 0.089 | 0.326 | ----- | ----- |
| Systolic BP (mmHg) | 0.550 | <0.001 | 0.148 | 0.332 |
| Diastolic BP (mmHg) | 0.641 | <0.001 | -0.304 | 0.043 |
| Fasting blood glucose (mg/dL) | 0.287 | 0.001 | -0.079 | 0.605 |
| Post prandial blood glucose (mg/dL) | 0.269 | 0.002 | -0.154 | 0.311 |
| HbA1c% | 0.585 | <0.001 | 0.007 | 0.444 |
| BUN (mg/dL) | 0.296 | 0.001 | -0.081 | 0.598 |
| Serum cholesterol (mg/dL) | 0.093 | 0.302 | -0.289 | 0.054 |
| Serum triglycerides (mg/dL) | 0.108 | 0.231 | -0.637 | 0.810 |
| ACR (mg/g) | 0.794 | <0.001 | -0.240 | 0.112 |
| eGFR (mL/min/1.73 m2) | -0.711 | <0.001 | 0.194 | 0.201 |
BMI: Body mass index; BUN: Blood urea nitrogen; ACR: Albumin creatinine ratio; eGFR: Estimated glomerular filtration rate (statistical significance was assessed by t test and inter group comparisons was done by ANOVA and post hoc analysis)
Twenty-three patients were in stage 1 Chronic Kidney Disease (CKD) (GFR ≥90 mL/min/1.73 sq.m), 58 patients in stage 2 (GFR 60-89 mL/min/1.73 sq.m), 28 patients in stage 3 (GFR 30-59 mL/min/1.73 sq.m) and 16 patients in stage 4 (GFR 15-29 mL/min/1.73 sq.m), while none in stage 5 CKD (GFR <15 mL/min/1.73 sq.m). On correlating the N-carboxymethyl lysine levels in patients with different stages of CKD, it increased with progressing stage of CKD.
The N-carboxymethyl lysine levels were significantly higher among the diabetic hypertensive patients than the diabetic non-hypertensive patients (1720±356 ng/L vs 1384±273 ng/L) with a p-value less than 0.001 and also expressed a significant positive correlation with both systolic and diastolic blood pressure. A positive correlation was also observed between the N-carboxymethyl lysine levels and patients’ age, BMI, duration of diabetes, fasting and Post Prandial Blood Sugars (PPBS), glycosylated haemoglobin (HbA1c), serum triglycerides, uACR and negative correlation with eGFR.
[Table/Fig-4] shows the correlation between serum carboxymethyl lysine levels and other diabetic complications. It is interesting to note that the serum levels of N-carboxymethyl lysine correlated positively and significantly with both retinopathy and left ventricular dysfunction apart from progressive stages of renal disease among the diabetic patients, which favours its correlation with diabetic vasculopathy.
Correlation between serum CML (carboxy-methyl lysine) levels and other diabetic complications.
| Characteristics | Serum CML (Carboxy-methyl lysine) (ng/L) |
|---|
| n | Mean±SD | p-value (Chi-square test) |
|---|
| Retinopathy | Present | 45 | 1857±352.44 | 0.005 |
| Absent | 80 | 1529±319.41 |
| LV dysfunction | Present | 62 | 1712±381.24 | 0.019 |
| Absent | 63 | 1594±347.26 |
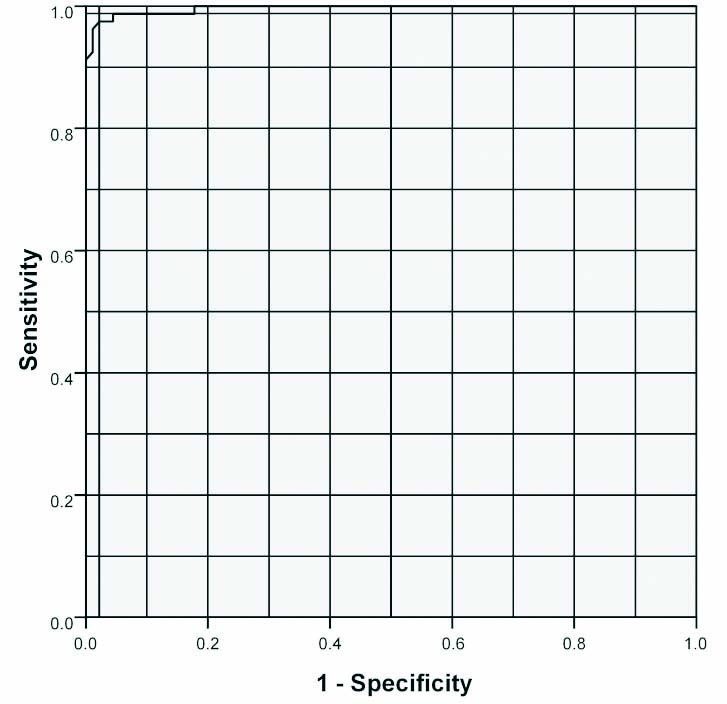
The Receiver Operating Characteristics (ROC) curve analysis of the study results is illustrated in the [Table/Fig-5], which demonstrates the diagnostic performance of N-carboxymethyl lysine in predicting the presence of diabetic nephropathy in patients with normoalbuminuria. The diagnostic performance of urinary podocin showed 97.5% sensitivity and 97.8% specificity with 97.5% positive predictive value and 97.7% negative predictive value with an area under curve at 0.997 at a cut-off value of 1455 ng/L.
Receiver Operating Characteristics (ROC) curve analysis with Area Under Curve (AUC) 0.997 at a cut-off value of 1455 ng/L.
Discussion
AGE products can be formed endogenously in the body or can be acquired from environment exogenously. Over the past century, studies have shown detrimental effects of these glycoproteins when accumulated in excess which includes diabetes, chronic pulmonary diseases, rheumatological and neurodegenerative diseases [21-24]. In diabetes the metabolism of excess glucose leads to glycolytic intermediates which increase the reactive aldehydes which further increases AGE products. Also, AGEs accumulation independent of hyperglycemia is well-established [25]. Oxidative stress among diabetic nephropathy patients further contributes to the formation of AGE [26]. Regardless of the sources, glycotoxins such as N-carboxymethyl lysine, methyl glyoxal derivatives and pentosidine have been shown to be significantly associated with the CKD progression.
The present study highlights the demographic clinical and laboratory characteristics, and identifies the risk factors involved in the progression of diabetic nephropathy. The present study establishes N-carboxymethyl lysine as an early non-invasive prognostic biomarker for DKD. In addition to this, older age, male sex, elevated blood pressure both systolic and diastolic were also found to be associated with progression from normoalbuminuria to macroalbuminuria among the diastolic patients [27].
A strong correlation was noted between BMI and AGE levels which probably indicate the risk for metabolic syndrome, as noted by Uribarri J et al., [28]. This stresses the importance of body weight in progression of diabetic complications.
In the index study, serum carboxymethyl lysine levels were found to be elevated in the normoalbuminuric patients and progressively increased from normoalbuminuric to microalbuminuric and further to the macroalbuminuric groups. Carboxymethyl lysine levels were significantly positively correlated with urinary ACR and creatinine while they displayed a significant negative correlation with GFR which suggest AGE elevation in early diabetic nephropathy marking the progression of DKD. This was slightly different from the observations noted by Wagner Z et al., who found such relationship only among diabetic patients with impaired renal function [29]. The inclusion of control group in the index study demonstrated a gradual increment in the carboxymethyl lysine levels across the study population as the disease progressed.
There has been emerging data that AGEs could be a key factor in the development of metabolic memory in diabetic complications and as its level is directly proportional to the duration of diabetes and degree of glycaemic control [30]. This is well-highlightened by the strong positive correlation between the serum AGE levels and glycosylated hemoglobin, fasting and post prandial glucose concentration in the different patient groups. However, in this study, serum AGE levels did not increase with increasing duration of diabetes.
Atherosclerosis has been well known to be associated with Diabetes Mellites, probably initiated by the human Low Density Lipoprotein Cholesterol (LDL-C) oxidation [31-34]. In the index study N-carboxymethyl lysine levels strongly positively correlated with total cholesterol, triglycerides and LDL. This favours AGEs as a potential marker for atherosclerosis [21] and atherosclerotic vasculopathy [32,33] among the diabetic patients and their correlation gradually increased with progressive nephropathy stage.
Earlier studies have demonstrated the association of elevated AGEs like glucosepane, methylglyoxal hydroimidazolone with many diabetic microvascular complications [35,36]. Semba RD et al., showed association between higher circulating carboxymethyl lysine levels and cardiovascular mortality [37] while Steine K et al., Hartog JW et al., and Galderisi M demonstrated association between elevated AGEs with both systolic and diastolic dysfunction in patients with diabetes mellites [38-40]. The index study shows a strong positive correlation between the AGE levels and left ventricular dysfunction.
ROC curve analysis was used to assess the diagnostic performance of serum N-carboxymethyl lysine. N-carboxymethyl lysine showed 97.5% sensitivity and 97.8% specificity to detect the nephropathy in the normoalbuminuric diabetic patients at a cut-off level of 1455 ng/L. Thus, it makes N-carboxymethyl lysine not just an early but a sensitive and specific biomarker for predicting the onset of nephropathy among the normoalbuminuric diabetic patients. This is in agreement with the observations of Hirata K and Kubo K favouring N-carboxymethyl lysine as a highly sensitive and specific marker to predict diabetic microangiopathy including nephropathy and retinopathy [41]. This is in contradiction to the observations made by Busch M et al., where they noted that N-carboxymethyl lysine did not predict renal outcomes in diabetic population [42]. This could be because of the difference in inclusion criteria of both the studies where patients on Angiotensin Converting Enzyme Inhibitors /Angiotensin Ii Receptor Blockers (ACEI/ARB) were excluded in the index study but in the study by Busch M et al., 455 patients with T2DM and nephropathy from the cohort of Irbesartan in diabetic nephropathy trial were followed-up [42].
Limitation(s)
The sample size was limited. Further evaluation of other AGE products in serum as well as skin by auto fluorescence and their correlation with the N-carboxymethyl lysine and comparison with other biomarkers of glycation or oxidation injury would have thrown more light on understanding the disease.
Conclusion(s)
N-carboxymethyl lysine seems to be a promising and early predictor of diabetic nephropathy which is also helpful in predicting the progression and severity of DKD among the type 2 DM population. Adequate glycaemic control, timely detection and managing higher blood pressure and controlling other metabolic complications will help in decreasing the progression of nephropathy. Thus, it can serve as a marker of diabetic vascular complications and can help in better management of the type 2 diabetes patients.
(BMI: Body mass index; FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; GFR: Glomerular filtration rate; LDL: Low density lipoprotein) (P1-between normoalbuminuric group and microalbuminuric group; P2-between normoalbuminuric group and macroalbuminuric group; P3-between microalbuminuric group and macroalbuminuric group, P4-between control group and normalbuminuric group; P5-between control group and microalbuminuric group; P6-between control group and macroalbuminuric group; Statistical analysis were done using student t-test and intergroup comparisons -ANOVA and post-hoc analysis)
FBS: Fasting blood sugar; PPBS: Post prandial blood sugar; GFR: Glomerular filtration rate; BUN: Blood urea nitrogen; LDL: Low density lipoprotein; uACR: Urinary albumin creatinine ratio; eGFR: Estimated glomerular filtration rate (student t-test and ANOVA were used to assess the statistical significance)
BMI: Body mass index; BUN: Blood urea nitrogen; ACR: Albumin creatinine ratio; eGFR: Estimated glomerular filtration rate (statistical significance was assessed by t test and inter group comparisons was done by ANOVA and post hoc analysis)
[1]. Parving HH, Oxenboll B, Svendsen PA, Christiansen JS, Andersen AR, Early detection of patienta at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretionActa Endocrinol (Copenh) 1982 100(4):550-55.10.1530/acta.0.10005506812342 [Google Scholar] [CrossRef] [PubMed]
[2]. Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H, Microalbuminuria as a predictor of clinical nephropathy in insulin dependent diabetes mellitusLancet 1982 1(8287):1430-32.10.1016/S0140-6736(82)92450-3 [Google Scholar] [CrossRef]
[3]. Mogensen CE, Christensen CK, Predicting diabetic nephropathy in insulin-dependent patientsN Engl J Med 1984 311(2):89-93.10.1056/NEJM1984071231102046738599 [Google Scholar] [CrossRef] [PubMed]
[4]. Williams ME, Diabetic nephropathy: The proteinuria hypothesisAm J Nephrol 2005 25(2):77-94.10.1159/00008428615746541 [Google Scholar] [CrossRef] [PubMed]
[5]. Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS, Regression of microalbuminuria in type 1 diabetesN Engl J Med 2003 348(23):2285-93.10.1056/NEJMoa02183512788992 [Google Scholar] [CrossRef] [PubMed]
[6]. Fioretto P, Steffes MW, Mauer M, Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuriaDiabetes 1994 43(11):1358-64.10.2337/diab.43.11.13587926312 [Google Scholar] [CrossRef] [PubMed]
[7]. Caramori ML, Fioretto P, Mauer M, Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesionsDiabetes 2003 52(4):1036-40.10.2337/diabetes.52.4.103612663477 [Google Scholar] [CrossRef] [PubMed]
[8]. The Diabetes Control and Complications Trial Research GroupNathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitusN Engl J Med 1993 329(14):977-86.10.1056/NEJM1993093032914018366922 [Google Scholar] [CrossRef] [PubMed]
[9]. Turner RC, Cull CA, Frighi V, Holman RR, Glycaemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) GroupJAMA 1999 281(21):2005-12.10.1001/jama.281.21.200510359389 [Google Scholar] [CrossRef] [PubMed]
[10]. Barbosa J, Steffes MW, Sutherland DE, Connett JE, Rao KV, Mauer SM, Effect of glycaemic control on early diabetic renal lesions. A 5-year randomised controlled clinical trial of insulin-dependent diabetic kidney transplant recepientsJAMA 1994 272(8):600-06.10.1001/jama.1994.035200800420418057515 [Google Scholar] [CrossRef] [PubMed]
[11]. Brownlee M, The pathobiology of diabetic complications: A unifying mechanismDiabetes 2005 54(6):1615-25.10.2337/diabetes.54.6.161515919781 [Google Scholar] [CrossRef] [PubMed]
[12]. Brownlee M, Biochemistry and molecular cell biology of diabetic complicationsNature 2001 414(6865):813-20.10.1038/414813a11742414 [Google Scholar] [CrossRef] [PubMed]
[13]. Ahmed N, Thornalley PJ, Advanced glycation endproducts: What is their relevance to diabetic complications?Diabetes Obes Metab 2007 9(3):233-45.10.1111/j.1463-1326.2006.00595.x17391149 [Google Scholar] [CrossRef] [PubMed]
[14]. Miura J, Yamagishi S, Uchigata Y, Takeuchi M, Yamamoto H, Makita Z, Serum levels of non-carboxymethyl lysine advanced glycation end products are correlated to severity of microvascular complications in patients with Type 1 diabetesJ Diabetes Complications 2003 17(1):16-21.10.1016/S1056-8727(02)00183-6 [Google Scholar] [CrossRef]
[15]. Yamagishi S, Matsui T, Advanced glycation end products, oxidative stress and diabetic nephropathyOxid Med Cell Longev 2010 3(2):101-08.10.4161/oxim.3.2.1114820716934 [Google Scholar] [CrossRef] [PubMed]
[16]. Ahmed N, Babaei-Jadidi R, Howell SK, Thornalley PJ, Beisswenger PJ, Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetesDiabetes Care 2005 28(10):2465-71.10.2337/diacare.28.10.246516186281 [Google Scholar] [CrossRef] [PubMed]
[17]. Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR, Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signalingCell Signal 2014 26(1):110-21.10.1016/j.cellsig.2013.10.00224113348 [Google Scholar] [CrossRef] [PubMed]
[18]. Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathyJ Clin Invest 1997 100(12):2995-3004.10.1172/JCI1198539399945 [Google Scholar] [CrossRef] [PubMed]
[19]. Zhou G, Li C, Cai L, Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathwayAm J Pathol 2004 165(6):2003-43.10.1016/S0002-9440(10)63254-3 [Google Scholar] [CrossRef]
[20]. Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, A new classification of diabetic nephropathy 2014: A report from Joint Committee on Diabetic NephropathyJ Diabetes Investig 2015 6(2):242-46.10.1111/jdi.1231925802733 [Google Scholar] [CrossRef] [PubMed]
[21]. Bucala R, Cerami A, Advanced glycosylation: Chemistry, biology, and implications for diabetes and agingAdv Pharmacol 1992 23:01-34.10.1016/S1054-3589(08)60961-8 [Google Scholar] [CrossRef]
[22]. Thorpe SR, Baynes JW, Role of the Maillard reaction in diabetes mellitus and diseases of agingDrugs Aging 1996 9(2):69-77.10.2165/00002512-199609020-000018820792 [Google Scholar] [CrossRef] [PubMed]
[23]. Miyata T, Ueda Y, Yamada Y, Izuhara Y, Wada T, Jadoul M, Accumulation of carbonyls accelerates the formation of pentosidine, an advanced glycation end product: Carbonyl stress in uremiaJ Am Soc Nephrol 1998 9(12):2349-56. [Google Scholar]
[24]. Münch G, Gerlach M, Sian J, Wong A, Riederer P, Advanced glycation end [24]products in neurodegeneration: More than early markers of oxidative stress?Ann Neurol 1998 44(3 Suppl 1):S85-88.10.1002/ana.4104407139749578 [Google Scholar] [CrossRef] [PubMed]
[25]. Ahmad S, Shahab U, Baig MH, Khan MS, Khan MS, Srivatsava AK, Inhibitory effect of metformin and pyridoxamine in the formation of early, intermediate and advanced glycation end-productsPLoS One 2013 8(9):e7212810.1371/journal.pone.007212824023728 [Google Scholar] [CrossRef] [PubMed]
[26]. Chilelli NC, Burlina S, Lapolla A, AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: A “glycoxidation-centric” point of viewNutr Metab Cardiovasc Dis 2013 23(10):913-19.10.1016/j.numecd.2013.04.00423786818 [Google Scholar] [CrossRef] [PubMed]
[27]. Perez-Hernandez J, Olivares MD, Solaz E, Martinez F, Martinez-Hervas S, Pichler G, Urinary podocyte associated molecules and albuminuria in hypertensionJ Hypertens 2018 36(8):1712-18.10.1097/HJH.000000000000174729677049 [Google Scholar] [CrossRef] [PubMed]
[28]. Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: A link between healthy and unhealthy obesity?J Clin Endocrinol Metab 2015 100(5):1957-66.10.1210/jc.2014-392525695886 [Google Scholar] [CrossRef] [PubMed]
[29]. Wagner Z, Wittmann I, Mazák I, Schinzel R, Heidland A, Kientsch-Engel R, N(epsilon)-(carboxymethyl) lysine levels in patients with type 2 diabetes: Role of renal functionAm J Kidney Dis 2001 38(4):785-91.10.1053/ajkd.2001.2769511576882 [Google Scholar] [CrossRef] [PubMed]
[30]. Singh R, Barden A, Mori T, Beilin L, Advanced glycation end-products: A reviewDiabetologia 2001 44(2):129-46.10.1007/s00125005159111270668 [Google Scholar] [CrossRef] [PubMed]
[31]. Stamler J, Vaccaro O, Neaton JD, Wentworth D, Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention TrialDiabetes Care 1993 16(2):434-44.10.2337/diacare.16.2.4348432214 [Google Scholar] [CrossRef] [PubMed]
[32]. Makita Z, Yanagisawwa K, Kuwajima S, Bucala R, Vlassara H, Koike T, The role of advanced glycosylation end-products in the pathogenesis of atherosclerosisNephrol Dial Transplant 1996 11(Suppl 5):31-33.10.1093/ndt/11.supp5.319044304 [Google Scholar] [CrossRef] [PubMed]
[33]. Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiencyProc Natl Acad Sci USA 1994 91(20):9441-45.10.1073/pnas.91.20.94417937786 [Google Scholar] [CrossRef] [PubMed]
[34]. Zyzak DV, Richardson JM, Thorpe SR, Baynes JW, Formation of reactive intermediates from Amadori compounds under physiological conditionsArch Biochem Biophys 1995 316(1):547-54.10.1006/abbi.1995.10737840665 [Google Scholar] [CrossRef] [PubMed]
[35]. Genuth S, Sun N, Cleary PA, Gao X, Sell DR, Lachin JM, Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with longterm microvascular complication progression of type 1 diabetesDiabetes 2015 64(1):266-78.10.2337/db14-021525187362 [Google Scholar] [CrossRef] [PubMed]
[36]. Monnier VM, Sell DR, Strauch C, Sun W, Lachin JM, Cleary PA, The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetesJ Diabetes Complicat 2013 27(2):141-49.10.1016/j.jdiacomp.2012.10.00423153673 [Google Scholar] [CrossRef] [PubMed]
[37]. Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L, Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adultsJ Am Geriatr Soc 2009b 57(10):1874-80.10.1111/j.1532-5415.2009.02438.x19682127 [Google Scholar] [CrossRef] [PubMed]
[38]. Steine K, Larsen JR, Stugaard M, Berg TJ, Brekke M, Jorgensen KD, LV systolic impairment in patients with asymptomatic coronary heart disease and type 1 diabetes is related to coronary atherosclerosis, glycaemic control and advanced glycation endproductsEur J Heart Fail 2007 9(10):1044-50.10.1016/j.ejheart.2007.07.01317719271 [Google Scholar] [CrossRef] [PubMed]
[39]. Hartog JW, Voors AA, Bakker SJL, Smit AJ, Veldhuisen DJ, Advanced glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implicationsEur J Heart Fail 2007 9(12):1146-55.10.1016/j.ejheart.2007.09.00918023248 [Google Scholar] [CrossRef] [PubMed]
[40]. Galderisi M, Diastolic dysfunction and diabetic cardiomyopathy: Evaluation by Doppler echocardiographyJ Am Coll Cardiol 2006 48(8):1548-51.10.1016/j.jacc.2006.07.03317045886 [Google Scholar] [CrossRef] [PubMed]
[41]. Hirata K, Kubo K, Relationship between blood levels of N-carboxymethyl lysine and pentosidine and the severity of microangiopathy in type 2 diabetesEndocrine Journal 2004 51(6):537-44.10.1507/endocrj.51.53715644571 [Google Scholar] [CrossRef] [PubMed]
[42]. Busch M, Franke S, Wolf G, Brandstädt A, Ott U, Gerth J, The advanced glycation end product N (epsilon)-carboxymethyl lysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertensionAm J Kidney Dis 2006 48(4):571-79.10.1053/j.ajkd.2006.07.00916997053 [Google Scholar] [CrossRef] [PubMed]