Genetic abnormalities can cause infertility in males by affecting the sperm production, sperm transport or rarely by affecting the hypothalamic-pituitary gonadal axis. Men with non-obstructive azoospermia and severe oligozoospermia (<5 million/mL) are at increased risk of having a genetic abnormality compared to fertile men [1,2]. Numerical and structural chromosomal abnormalities that affect the testicular function and YCMD that cause spermatogenic impairment are seen frequently in men with azoospermia and severe oligospermia. Obstructive azoospermia mostly caused by CFTR gene mutations affecting the internal ductal system, genes affecting sperm function and other non-specific disorders are other genetic causes.
The overall incidence of chromosomal factor in infertile males ranges from 2 to 10% [3]. In comparison, in 94,465 newborn males the incidence of chromosomal abnormalities was 0.39%, of which, sex chromosomal abnormalities comprised 0.14% and autosomal abnormalities 0.25% [3]. The incidence of chromosomal abnormalities in azoospermia and oligozoospermia reported in the literature varied from 13.1% to 23.1% to 2.1% to 6.6%, respectively [3-5]. Van Assche E et al., in a study of 694 infertile men found that chromosomal abnormalities were eight times more frequent in infertile men when compared to fertile men [3]. Retief AE et al., found an overall prevalence of 7.1% and in 14.1% of the azoospermia and 5.1% of the oligozoospermia group and therefore constitutional chromosomal aberrations increase as sperm counts decrease [6]. It is also found that incidence of numerical sex chromosomal aberrations are proportionally higher in males with azoospermia whereas structural chromosomal aberrations of autosomes such as Robertsonian and reciprocal translocations are proportionately more frequent in oligospermic males [7,8].
Understanding the genetic aetiology of severe male factor infertility is imperative for patient counseling and decision making considering the advances in sperm retrieval techniques and sperm cryopreservation relating to increased success rates with ICSI.
The present study was conducted with an aim to determine the prevalence of abnormal karyotypes among men with azoospermia and severe oligzoospermia (<5 million/mL).
Materials and Methods
The present study was a retrospective observational study conducted at the Fertility clinic, Institute of Maternal and Child Health, Medical College, Calicut, Kerala, India. Semen analysis was done two times in one month interval and 232 patients with azoospermia and oligozoospermia, 100 and 132, respectively, who attended the Infertility Department between January 2016 to December 2019 were analysed. The study protocol was approved by the Institutional Ethics Committee (IEC-537). Written/informed consent from the study participants was obtained.
Inclusion and Exclusion criteria: Males having semen analysis suggestive of azoospermia i.e., no sperms found after centrifugation in two semen samples, after excluding obstructive pathologies and males having semen analysis suggestive of severe oligospermia with a sperm concentration of <5 million/mL were included in the study [1]. Those with clinical evidence of obstructive azoospermia were excluded.
These patients underwent a physical examination, seminogram and basic hormone profile (Follicle Stimulating Hormone (FSH), Testosterone). A detailed pedigree analysis was done in all patients and after genetic counselling, venous blood samples (Five mL) were taken into heparinised test tubes from each patient. Karyotyping was done from the Cytogenetics Unit, Department of Anatomy. The samples were cultured for 72 hours using PB Max karyotyping medium. At the end of 72 hours, the cultures were harvested and G-T-G banding was performed. The metaphases were analysed by Cytovision 7 software. Twenty metaphases were counted, karyotyped and analysed as per the standard lab protocol followed in cytogenetic laboratory. In the case of any abnormality the metaphases analysed were increased to 30-50 [9].
Statistical Analysis
The data was entered in MS excel sheet. The prevalence of different autosomal and sex linked chromosomal aberrations among azoospermic and severe oligospermic patients was studied and expressed in percentage.
Results
Karyotype was done in 100 patients with azoospermia and 132 cases of oligospermia. The patients were between 26 and 45 years of age. Chromosomal abnormalities were detected in 35 (35%) of 100 azoospermia and 15 (11.3%) of 132 severe oligospermia cases analysed [Table/Fig-1]. A total of 28 (80%) of azoospermia cases were gonosomal and 7 (20%%) were autosomal. In oligozoospermia 4 (26.7%) were gonosomal and 11 (73.3%) were autosomal.
Frequency of chromosomal abnormalities detected.
| Semen analysis | Number | Abnormal karyotype | % |
|---|
| Azoospermia | 100 | 35 | 35% |
| Oligozoospermia | 132 | 15 | 11.3% |
| Total | 232 | 50 | 21.5% |
The various chromosomal abnormalities detected in azoospermia are given in [Table/Fig-2].
Karyotype abnormalities in azoospermia (35).
| Abnormality | Total number | Type of abnormality | No. of cases | % (of total) |
|---|
| Numerical abnormalities | 22 | Klinefelter syndrome | 22 | 9.5% (22/232) |
| Structural abnormalities | 3 | Inversiona) 46XY inv 9 (p13q13)Derivativesa) 46XY der chr 1b) 46 X, der X | 111 | 1.3% (3/232) |
| Polymorphic variants | 5 | 46XY 15pstk+46XY 15ps+46XY 1qh+46XY 9qh+ | 1211 | 2.1% (5/232) |
| Others | 2 | 46XX | 2 | 0.8% (2/232) |
| Small Y | 3 | 46XYY microdeletion | 3 | 1.3 (3/232) |
| Total | | | 35 | 15% (35/232) |
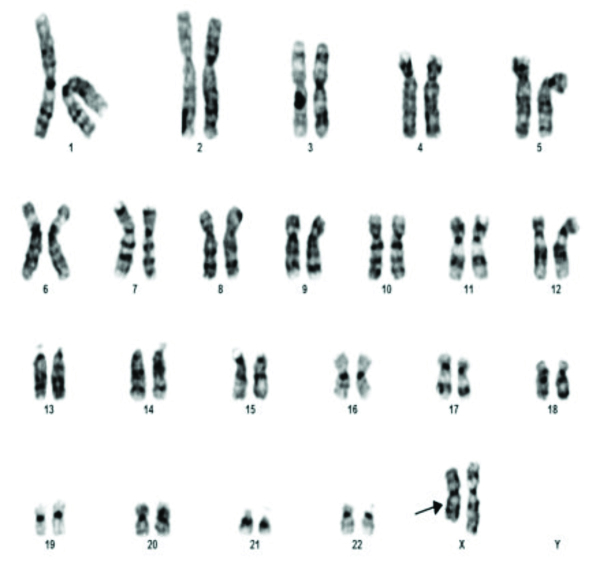
The commonest abnormality found in azoopsermia was Klinefelter syndrome (22/35). Structural abnormalities detected were 46X der X, 46XY der Chr 1 and Chromosome 9 inversion [Table/Fig-3] in one case each. Polymorphic variants were found in five cases. 46XY 15ps+ [Table/Fig-4] with enlarged satellite of acrocentric chromosome 15 in three patients, 9q polymorphism and 1q polymorphism in one patient each. 46XX [Table/Fig-5] was found in two patients. Small Y-Y chromosome [Table/Fig-6] was found in three patients.
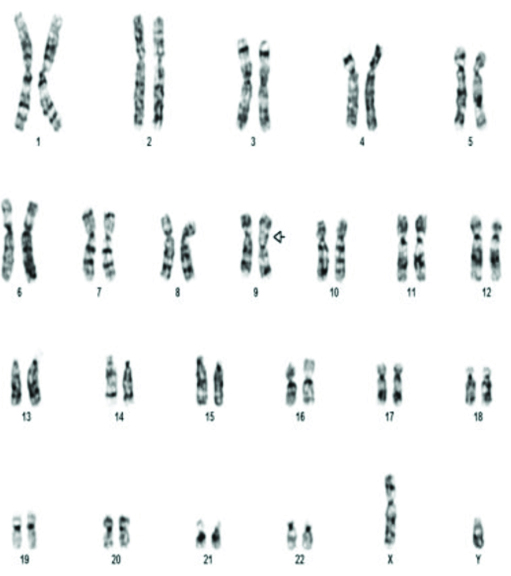
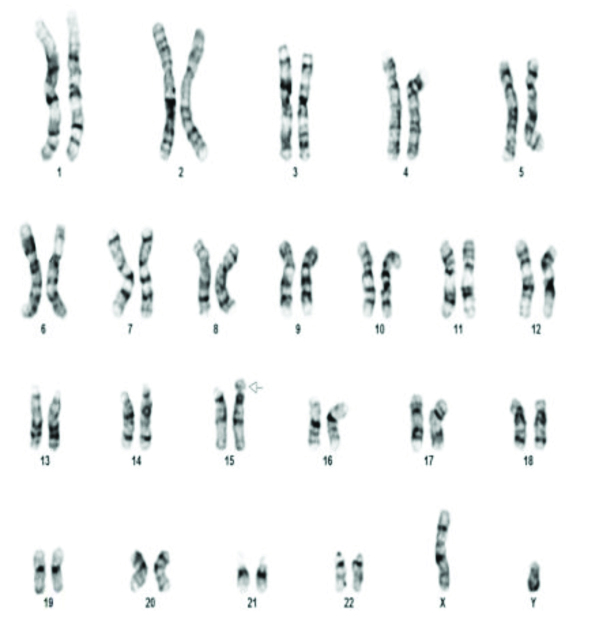
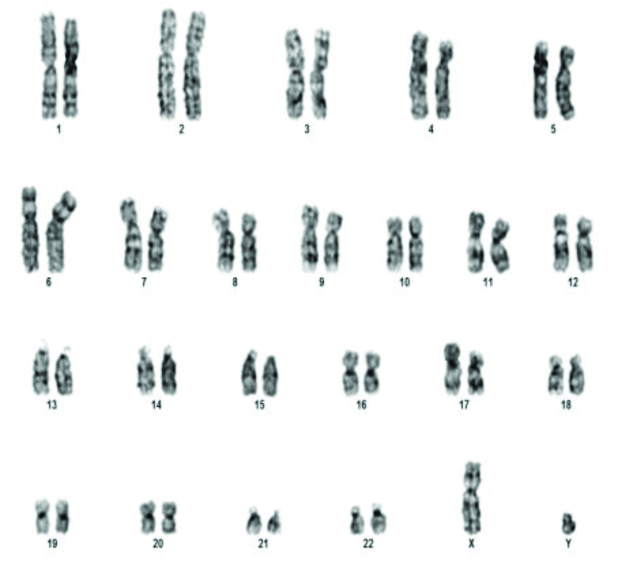
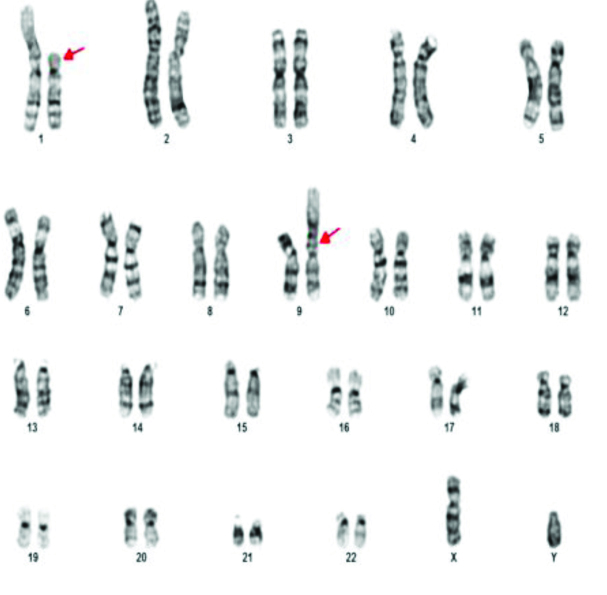
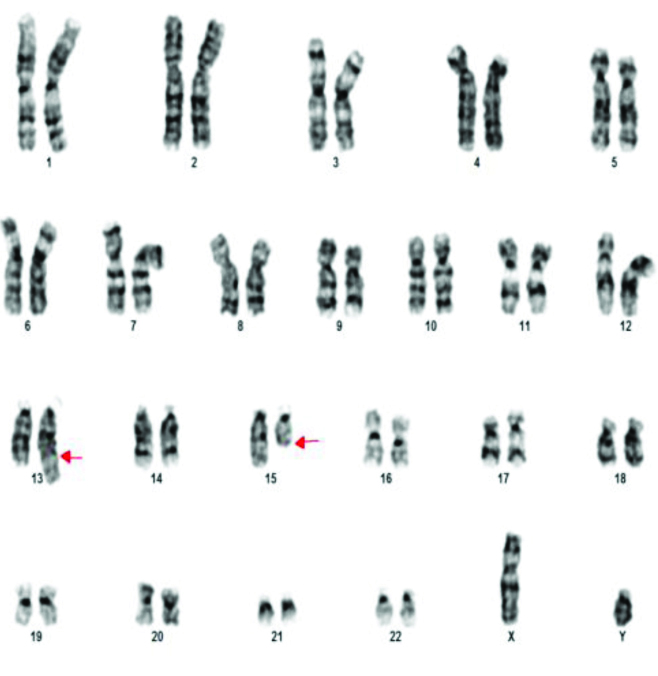
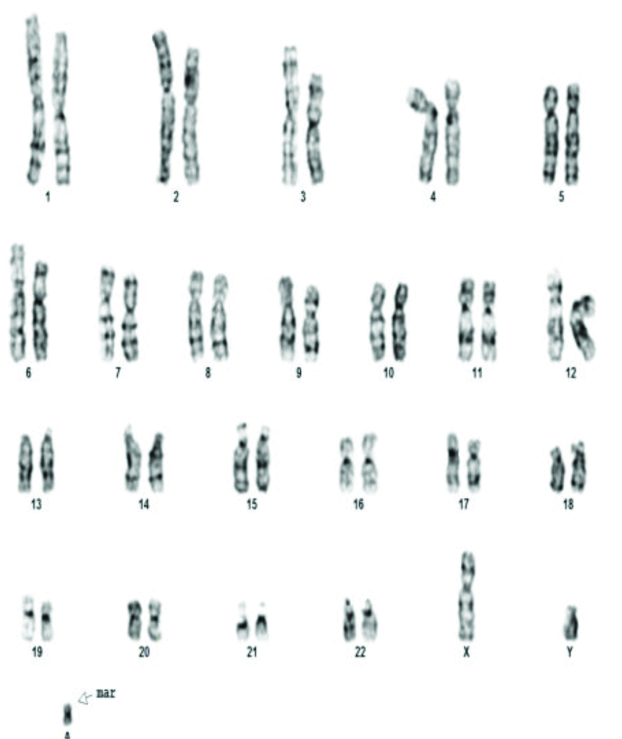
The various chromosomal abnormalities detected in oligozoospermia are given in [Table/Fig-7]. Klinefelter syndrome was found in two patients. One patient was found to have 48XXYY [Table/Fig-8]. Structural abnormalities were found in six cases. Translocations in four patients 46XY,t(11;13)(q21;q21.2) [Table/Fig-9], 46XY,t(1;9)(p13;p21) [Table/Fig-10], 46XY,t(13;15)(q34;q21) [Table/Fig-11]. Der 15 in one and marker chromosome [Table/Fig-7,12] in one patient each.
Karyotype abnormalities in severe oligozoospermia (15).
| Abnormality | Total number | Type of abnormality | No: of cases | % (of total) |
|---|
| Numerical abnormalities | 3 | a) Klinefelter syndromeb) 48XXYY | 21 | 1.3%(3/232) |
| Structural abnormalities | 6 | Translocationsa) 46XY,t(11;13)(q21;q21.2)b) 46XY,t(1;9)(p13;p21)c) 46XY,t(13;15)(q34;q21)d) 46XY,t(7,14)(q34:q11)Derivatives46XY der 15Marker chromosome47XY+mar | 411 | 2.6%(6/232) |
| Polymorphic variations | 5 | 46XY 21pstk46XY 15ps+46XY 1qh-48XY 9qh+ | 1121 | 2.1%(5/232) |
| Small Y | 1 | 46XY Y microdeletion | 1 | 0.4%(1/232) |
| Total | | | 15 | 6.4%(15/232) |
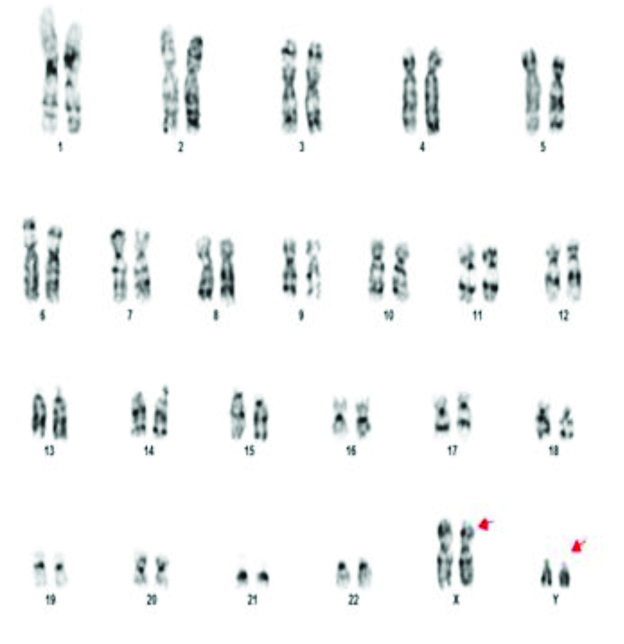
46XY,t(11;13)(q21;q21.2).
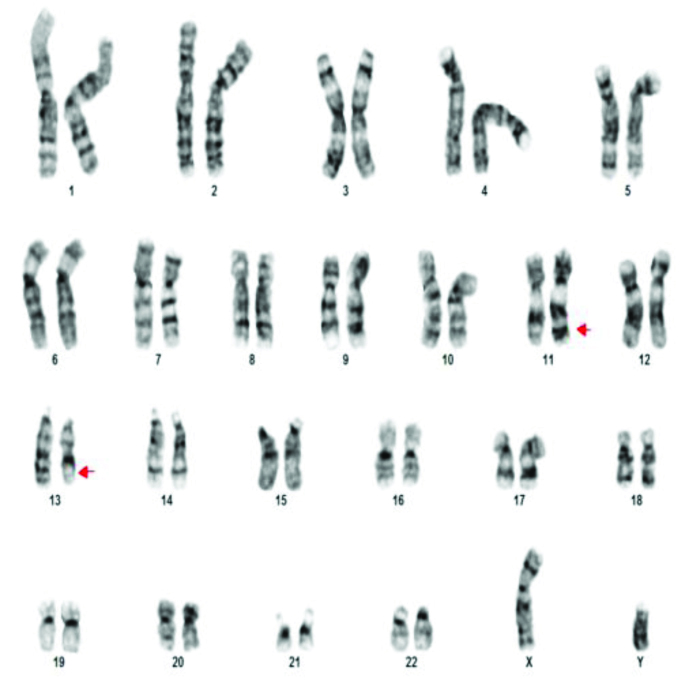
A 46XY,t(13;15)(q34;q21).
Discussion
In this study, the incidence of chromosomal abnormalities was higher in azoospermia, 35% compared to 11.3% in severe oligospermia. The results of this study were in concordance with a study by Xie C et al., in which, in 912 patients, 27.3% (146/534) of azoospermia and 15.9% (60/378) of severe oligozoospermia had genetic abnormalities [10]. In a study of 744 South Indian infertile men, Lakshmi Rao K et al., found that 11% and 6.1% incidence of chromosomal abnormalities in azoospermia and severe oligozoospermia respectively and sex chromosomal abnormalities were more frequently associated with azoospermia [8]. Similar results were observed in a study by Yatsenko AN et al., in a study of 668 infertile males [11]. The higher incidence of chromosomal abnormalities in present study could be because polymorphic variants were considered to be abnormal whereas in others, they are considered to be normal variations.
Gonosomal abnormalities were more frequently associated with azoospermia (80%) whereas autosomal aberrations predominated in non-azoospermic men (73.3%) in present study. This was found to be 83% and 65% in a study of 1223 infertile men by Dul EC et al., [12].
In this study, of the 35 chromosomal abnormalities found in men with azoospermia, 22 had Klinefelter syndrome. It was also found in two cases of severe oligospermia. Klinefelter syndrome is observed in about 10-15% of cases with azoospermia and up to 5% in severely oligozoospermic men while it occurs in approximately 0.1-0.2% of newborn males [13,14]. The typical Klinefelter male presents with spermatogenic and androgenic failure but phenotypic presentations may be variable and some have normal androgenisation. About 80% of the Klinefelter men has 47,XXY karyotype while 20% are mosaics (46,XY/47,XXY) [15].
A rare chromosomal abnormality, 46 XX, found in 0.9% of the azoospermic males [16] was found in 2 (0.8%) phenotypic males presenting with azoospermia in this study.
Out of 232 cases, one patient with severe oligospermia showed 48XXYY karyotype. He was phenotypically male with small sized testes. A 48XXYY syndrome causes infertility, developmental and behavioural problems. This syndrome affects one male in every 18000-40000 live births [17]. No case of mosaic Klinefelter was found in present study. Mosaic Klinefelter syndrome have more chance of retrieving testicular spermatozoa [18]. In 48XXYY syndrome, the extra sex chromosome almost always comes from a sperm. Non-disjunction may cause a sperm to gain two extra sex chromosomes resulting in a spermatozoon with three sex chromosomes (one X and two Y), when this sperm is fertilised with a normal oocyte with one X chromosome, the resulting zygote will have 48 XXYY complement. In patients with severe oligozoospermia, in this study, structural autosomal abnormalities were more frequent than sex chromosome abnormalities. Chromosomal translocations found, were those involving chromosomes 1,7,9,11,13, 14 and 15. There were no cases of translocation in men with azoospermia in this study.
Robertsonian translocations feature centric fusion of two non-homologous acrocentric chromosomes (13, 14, 15, 21, and 22). The most common translocations involve chromosomes 13;14 and 14;21 [18]. These translocations occur in 0.2% of azoospermia cases and in 1.5% of oligozoospermic males [19]. Translocation carriers have varying fertility status. Following Invitro Fertilisation (IVF)/ICSI, the risk of foetus with unbalanced translocation is low (<1%) in 13;14 carriers [20]. Non-disjunction can lead to a viable trisomic child or after postzygotic correction, to a child with uniparental disomy [21].
Reciprocal translocations in which material is exchanged between any two chromosomes can occur in 0.7% of infertile males. The risk of meiotic imbalance depends on the chromosomes involved and the breakpoint positions. As there is high risk of sperm aneuploidy Preimplantation Genetic Diagnosis (PGD) is recommended to implant balanced embryos [22].
Chromosome 9 inversions are found in 3-5% of the infertile men [23]. It was found in one patient with azoospermia in present study. Male carriers of chromosome 9 inversions may have infertility due to spermatogenic disturbances caused by the loops or acentric fragments [24,25].
Polymorphic variants are known to occur in the general population. Though they have no impact on phenotype higher frequencies of these variants are found in infertile and subfertile individuals [26]. These have been associated with poor spermatogenesis due to the effect of these heterochromatic variations on genes controlling spermatogenesis [27]. A 2.1% of the azoospermic and oligospermic patients were found to have polymorphisms involving chromosome 21,15, 9 and 1 in this study. Human chromosome 9 is highly polymorphic and contains the largest autosomal block of heterochromatin. Enlarged chromosome 9 heterochromatin (9qh+) is considered a polymorphism, and it occurs in 6% and 8% of the population. In a study of teratozoospermic men Eiben B et al., found 9qh+ in 25% of the patients and though it does not have phenotypic effects it was associated with infertility and reproductive failure [28].
Nakamura Y et al., in a study of 1790 infertile Japanese men found that 46,XY,1qh(+) was the commonest autosomal anomaly, observed in 30 cases, nine of whom had azoospermia [29]. In a study of 392 infertile men by Christofolini DM et al., the variant 1qh+ was associated with other variations (Yqh+, 16qh+) and the patients presented with oligozoospermia or infertility [30]. Nagvenkar P et al., also found an association between with azoospermia and 16qh+ and 1qh+ variants [31].
Marker chromosome was found in one patient with oligospermia in present study. As Fluorescence In Situ Hybridization (FISH) was not available the nature of the marker chromosome could not be identified. Small supernumerary marker chromosomes has been reported to occur in 0.125% of infertile males [32]. The most common Supernumerary Marker Chromosome (sSMC) in humans is sSMC [15]. It is currently thought that it could disrupt the chromosome movements during meiosis and cause oligospermia [33].
Small Y was found in 1.3% (three) of azoospermia and 0.4% (one) of oligospermia cases in this study. This may suggest YCMD involving the azoospermia factor (AZF) region of the Y chromosome which contains genes critical for spermatogenesis. YCMD are found in 10% of men with non-obstructive azoospermia and 5% of those with oligospermia [34].
The results of this study shows the high incidence of chromosomal abnormalities in men with azoospermia and severe oligozoospermia and karyotyping before IVF/ICSI is recommended in these group of patients. In addition to making a definitive diagnosis of the aetiology of their infertility, detection of Klinefelter syndrome allows for androgen therapy and follow-up for cardiovascular diseases, diabetes and osteoporosis which are known to occur with an increased frequency [35]. The chances for sperm retrieval in indicated cases can be predicted by the nature of the chromosomal abnormalities. ICSI bypasses the natural selection process and can cause recurrent implantation failure or miscarriages and detailed counseling regarding implications on fertility and need for preimplantation genetic diagnosis is mandatory.
With Y chromosome microdeletion analysis, which was not available in our institution more genetic causes could have been detected. Novel techniques like spermatozoal FISH, Single sperm Polymerase Chain Reaction (PCR), meiotic analysis of synaptonemal complex etc., can provide more insight into the chromosomal abnormalities that can affect fertility.
Limitation(s)
Correlation of anthropometric measurements, testicular volume, FSH and testosterone levels with chromosomal abnormalities would have helped to better predict the chance of finding abnormal karyotypes in men with azoospermia and severe oligozoospermia.
Conclusion(s)
The results of this study highlight the importance of cytogenetic studies in men with azoospermia and severe oligospermia. The presence of chromosomal abnormalities provide a definitive diagnosis and can guide the further treatment protocols.