There is increasing anecdotal evidence that olfactory and gustatory dysfunction may be associated with SARS-CoV-2 and they may potentially be the early symptoms of the disease [10]. Smell and taste dysfunctions have been known to be associated with various other viral infections, such as rhinovirus, Epstein-Barr virus and certain other strains of coronavirus like Severe Acute Respiratory Syndrome Coronavirus-1 (SARS-CoV-1) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV). These viruses cause inflammation of nasal passages leading to rhinorrhoea, nasal obstruction and olfactory dysfunction [11,12]. Evidence suggests that unlike other viruses, olfactory dysfunction in COVID-19 may not present with co-existing rhinorrhoea and nasal obstruction [6].
Walker A et al., used Google trends to analyse the number of Google searches made, related to smell in multiple countries. They found a strong correlation between smell related searches and onset of COVID-19 infections, hypothesising that smell dysfunction may be an under-recognised symptom of COVID-19 [13]. In view of emerging evidence of smell and taste dysfunctions in COVID-19, the American Association of Otorhinolaryngology and Head and Neck Surgery has proposed to include anosmia, hyposmia and dysgeusia as screening tools for detection of COVID-19 [14].
A number of studies across the world have reported the prevalence of smell and taste dysfunctions such as anosmia, hyposmia, dysgeusia and ageusia in association with COVID-19 [6,15-29]. Few more have also established a strong association between smell and taste dysfunction and COVID-19 [23,30-32]. This systematic review and meta-analysis estimated the association of olfactory and gustatory dysfunctions with COVID-19 and their pooled prevalence across the studies [23]. To author’s knowledge, this is the first meta-analysis assessing the association of smell and taste dysfunction with COVID-19.
Materials and Methods
Protocol and registration: This systematic review and meta-analysis was done according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) and Meta-Analyses of Observational Studies in Epidemiology (MOOSE) guidelines. The protocol for this systematic review was designed and registered with PROSPERO (Registration Number- CRD42020181841) [33]. The review was conducted in May, 2020 and extended over a study period of two months.
Search strategy: The following databases were searched: PubMed, Embase, EBSCO and Cochrane Central Register of Controlled Trials (CENTRAL) database. The searches were supplemented by screening references of the included studies. Respective authors were also contacted for a few unclear data. The primary search period included all studies published until 1st May 2020, without language restrictions. The following strategy was used to search the MEDLINE database: [[“COVID-19”[All Fields] OR “COVID-2019”[All Fields] OR “severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “2019-nCoV”[All Fields] OR “SARS-CoV-2”[All Fields] OR “2019nCoV”[All Fields] OR [[“Wuhan”[All Fields] AND [“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields]]] AND [2019/12[PDAT] OR 2020[PDAT]]]] OR [“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields]] OR [“severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “sars cov 2”[All Fields]]] AND [[“olfaction disorders”[MeSH Terms] OR [“olfaction”[All Fields] AND “disorders”[All Fields]] OR “olfaction disorders”[All Fields] OR “anosmia”[All Fields]] OR hyposmia[All Fields] OR [“dysgeusia”[MeSH Terms] OR “dysgeusia”[All Fields]] OR [“ageusia”[MeSH Terms] OR “ageusia”[All Fields]] OR [“taste”[MeSH Terms] OR “taste”[All Fields]] OR [“smell”[MeSH Terms] OR “smell”[All Fields]] OR [“taste”[MeSH Terms] OR “taste”[All Fields] OR “gustation”[All Fields]]]. The search strategy for CENTRAL, EBSCO and EMBASE were similar to those used in the MEDLINE search.
Selection of studies: Reference management software (Zotero 5.0.85) was used to save all the potentially eligible studies. Two reviewers (KP and SP) independently screened the available searched studies for inclusion by reviewing the title and abstracts. Any disagreements amongst the two reviewers were resolved by involving a third reviewer. In cases where abstracts did not provide adequate or relevant information, full texts of papers were screened for eligibility. Following this, the full text of all included studies was retrieved and reviewed in detail. Excluded studies were stated with the reasons for their exclusion.
Selection criteria: All cross-sectional, cohort and case-control studies evaluating olfactory and gustatory symptoms in patients with COVID-19 were included. Studies published on or before 1st May 2020 were included. Case reports, case-series, narrative reviews and letters to the editor were excluded. Studies with insufficient data regarding olfactory and gustatory symptoms were excluded.
Data collection and synthesis of results: Two reviewers independently extracted relevant information. Tabulation of the following data was done in Microsoft Excel sheet: First author, publication date, study design, country, number of COVID-19 positive patients, mean or median age of patients, gender, total patients with olfactory dysfunction, total patients with gustatory dysfunction, patients with combined olfactory and gustatory dysfunctions (both), patients with olfactory or gustatory dysfunctions as presenting symptoms, patients with anosmia, hyposmia, ageusia, hypogeusia, dysgeusia, smell recovery time and any significant associations between the variables.
Assessment of study quality: The quality assessment tool by the National Institutes of Health (NIH) for observational studies and case-control was used to assess the quality of included studies [34]. Zero or 1 point was scored for each item according to the criteria and overall quality score was generated by adding the individual scores for each item. Based on the scores, the studies were classified as having good quality (≥7), fair quality (5-6) and poor quality (≤4). Good quality studies are deemed to have least risk of bias. Studies with fair quality have moderate amount of bias whereas poor quality studies have a significant risk of bias [35].
Statistical Analysis
The data was analysed using STATA 13.0 (StataCorp, College station, TX) and Revman version 5.3. Random effects model was used to calculate a pooled OR to determine the overall association between Olfactory and Taste Disorder (OTD) and COVID-19. Using random effects model, pooled prevalence of olfactory dysfunction, gustatory dysfunction, patients with both olfactory and gustatory dysfunction, and patients with some form of chemosensory dysfunction (taste and/or smell) were assessed. Apart from these, pooled prevalence of different types of smell and taste disturbances such as anosmia, hyposmia, ageusia and dysgeusia were calculated. Heterogeneity of the studies was calculated using I2 statistics (Higgins JPT et al.,) [36].
Results
Study selection: The records identified 140 articles by the application of the search strategy. After removing the duplicate search records, a total of 74 records were identified from the electronic searches [Table/Fig-1].
Study selection process illustrated by preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
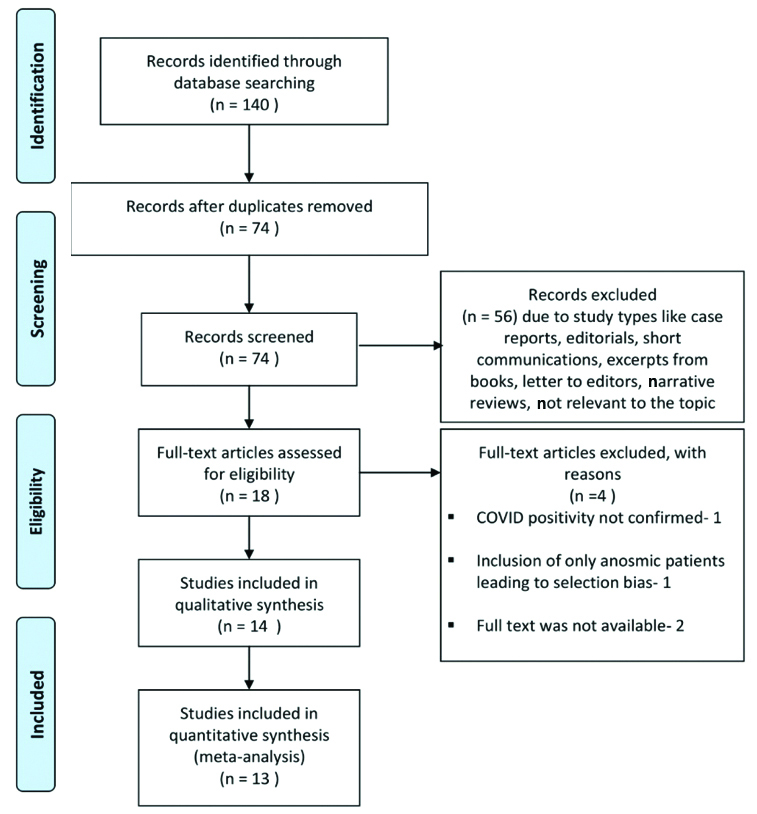
These search records were screened for title and abstracts. Three articles did not had an English translation of which one was in Danish, one in German and one in Greek. Fifty three records were excluded due to publication types like case reports (2), case series (2), editorials (5), short communications (1), excerpts from books (1), letter to editors (5), narrative reviews (6) and due to irrelevance to the topic (31). For the remaining 18 records, full text articles were searched for eligibility. Of these, four articles were excluded due to the following reasons: COVID-19 positivity was not confirmed in one study, one study included only anosmic patients leading to selection bias and full text was not available for two articles. Thus, 14 [6,16,17,21-23,27,30-32,37-40] studies were included in qualitative synthesis and 13 studies were incorporated in quantitative synthesis. Of these 11 were used to measure the pooled prevalence, and 5 [23,27,30-32] studies were used to establish an overall association by measuring pooled OR.
Study characteristics: Fourteen studies consisting of six cross-sectional surveys [6,16,17,37,39,40], three retrospective observational studies [21,22,38], five case-control studies [23,27,30-32] with a total of 3,125 patients included in the final review [Table/Fig-2] [6,16,17,21-23,27,30-32,37-40].
Table describing the study characteristics of the included studies [6,16,17, 21-23,27,30-32,37-40].
| S. No. | Author | Country | Age (Years) Mean (SD)/Median | COVID Positive cases | Male | Female | Study design | Patients with olfactory dysfunction (%) | Patients with gustatory dysfunction (%) |
|---|
| 1 | Giacomelli A et al., [16] | Italy | Median 60 | 59 | 40 | 19 | Cross-sectional survey | 14 (23.7) | 17 (28.8) |
| 2 | Lechien JR et al., [6] | Europe | 36.9 (11.4) | 417 | 154 | 263 | Cross-sectional survey | 357 (85.6) | 342 (82.01) |
| 3 | Yan CH et al., [38] | USA | 18-79 | 59 | 29 | 29 | Cross-sectional survey | 40 (67.8) | 42 (71.2) |
| 4 | Klopfenstein T et al., [22] | France | 47 (16) | 114 | Not reported | Not reported | Retrospective observational study | 54 (47) | 46 (85) |
| 5 | Mao L et al., [21] | China | 52.7 (15.5) | 214 | 87 | 127 | Retrospective observational study | 11 (5.1) | 12 (5.6) |
| 6 | Moein ST et al., [23] | Iran | 46.55 (12.17) | 60 | 40 | 20 | Case-control study | 59 (98) | 14 (23.3) |
| 7 | Benezit F et al., [27] | France | Not reported | 68 | Not reported | Not reported | Case-control study | 31 (45) | 42 (62) |
| 8 | Vaira LA et al., [39] | Italy | 49.2 | 72 | 27 | 45 | Cross-sectional | 86.1 | 47.2 |
| 9 | Spinato G et al., [40] | United Kingdom | Median 56 | 202 | 97 | 105 | Cross-sectional survey | Not mentioned | Not mentioned |
| 10 | Lechein JR et al., [37] | Europe | 39.17 (12.09) | 1420 | 458 | 962 | Cross-sectional | 997 (70.2) | 770 (54.2) |
| 11 | Beltran- Corbellini A et al., [30] | Spain | 61.6 (17.4) | 79 | 48 | 31 | Case-control study | 25 (80.6) | 28 (90.3) |
| 12 | Tostmann A et al., [31] | Netherland | Not reported | 79 | 19 | 71 | Case-control study | 37 (46.8) | Not mentioned |
| 13 | Wee LE et al., [32] | Singapore | Not reported | 154 | Not reported | Not reported | Case-control study | Not mentioned | Not mentioned |
| 14 | Yan CH et al., [17] | USA | 53.5: Admitted patients, 43: Ambulatory patients | 128 | 61 | 67 | Retrospective observational study | 75 (58.5) | 70 (54.6) |
SD: Standard deviation
Assessment of the quality of the studies: The study quality was assessed using National Heart Lung and Blood Institute (NHLBI) quality assessment tool for observational studies by the NIH for the nine observational studies and the NHLBI tool for case-control studies was used for the five included case-control studies. The study qualities were good for nine studies, fair for four studies and poor for one study [Table/Fig-3].
Table describing the key findings, strengths and limitations of the studies [6,16,17,22-24,27,30-32,37-40].
| S. No. | Author/Year | Key features | Authors conclusion | Strengths | Limitations | Study quality |
|---|
| 1 | Giacomelli A et al., [16] March 2020 | 33.9% had at least one gustatory/olfactory disorder and 18.6% had both. 20.3% presented as initial symptoms. Taste dysfunction was more as an initial symptom (91%) Females had these symptoms more than males {52.6% versus 25%, p=0.036}. Patients with at least one olfactory/gustatory symptom were younger than those without
| Olfactory and gustatory dysfunction are frequent in patients with COVID-19. It may precede the onset of more serious disease.
| Patients with memory disorders were excluded
| Not used a structured questionnaire Subjective assessment Since the study was done for hospitalised patients, patients with mild symptoms who does not need hospitalisation may be missed Olfactory recovery not assessed
| Fair |
| 2 | Lechien JR et al., [6] April 2020 | Patients with mild-to-moderate disease 85.6% had olfactory dysfunction 88.0% of patients had gustatory dysfunctions. There was a significant association between both dysfunctions (p<0.001). Olfactory dysfunction was a presenting symptom in 11.8% cases. Quality of Life (QOL) scores were significantly lower in anosmia Majority olfactory dysfunctions were without nasal obstruction or rhinorrhea. Early olfactory recovery rate was 44.0%. Females were significantly more affected by smell and taste dysfunctions than males (p=0.001).
| In European COVID-19 patients, olfactory and gustatory dysfunctions were prevalent The sudden anosmia/ageusia should be recognised as a symptom of COVID-19 disease.
| Standard questionnaire Included varied ethnicity Mild to moderate cases included. QOL assessed Smell recovery assessed
| Subjective assessment Not included severe patients May not be representative as physicians with anosmia voluntarily participated.
| Good |
| 3 | Yan CH et al., [38] April 2020 | Anosmia and ageusia was reported in 68% and 71% patients, respectively (p<0.001). Anosmia and ageusia was independently and strongly associated with Covid-19 (anosmia: adjusted Odds Ratio (aOR) 10.9, 95% CI: 5.08-23.5; ageusia: aOR 10.2 95% CI: 4.74-22.1) 74% of anosmia patients recovered in two weeks with overall recovery from illness. 22% reported anosmia as an initial symptom Anosmia and ageusia were 10 times more common in Covid-19-positive patients
| Olfactory and gustatory dysfunction was strongly associated with COVID-19. Should be considered as a screening symptom Most of these symptoms recovered with overall recovery from illness
| 10 point olfaction score was used. Smell recovery assessed
| Subjective assessment Short sampling period Only ambulatory patients with relatively mild COVID-19 infection were included Moderate to severe patients admitted in the hospitals were not included Patients with memory disorders were not excluded
| Good |
| 4 | Klopfenstein T et al., [22] April 2020 | 47% had anosmia. Anosmia was associated with dysgeusia in 85% cases. Patients with anosmia were younger with a female predominance Anosmia started 4.4 {±1.9 (1-8)} days after onset of infection. Mean duration of anosmia - 8.9 {±6.3 (1-21)} days. 80% patients recovered in 14 days & 98% patients recovered within 28 days.
| Nearly half of COVID-19 patients had anosmia and it is associated with dysgeusia in 85% cases. Favourable outcome in 28 days.
| Smell recovery assessed Patients with memory disorders were excluded
| Data was retrospectively collected from the file where mild patients may not be captured Only taken hospitalised patients and ambulatory patients with these symptoms may be missed
| Fair |
| 5 | Villalba NL, et al., [24] April 2020 | Neurologic symptoms was noted in 36.4% patients These were common in patients with severe infection (45.5%) Amongst the peripheral nervous symptom symptoms, Gustatory dysfunction seen in 5.6% of which 3 had severe infection and 9 had nonsevere infection Olfactory dysfunction was seen in 5.1% patients of which 3 had severe infection and 8 had nonsevere infection
| Neurologic symptoms are seen in a sizable proportion of COVID-19 patients. Physicians should suspect COVID-19 as a differential diagnosis, if neurologic manifestations present
| Categorised and included both severe and nonsevere infection patients
| Data collected retrospectively collected where mild patients may not be captured Included only hospitalised patients Smell recovery not assessed
| Poor |
| 6 | Moein ST et al., [23] April 2020 | 98% patients had some form of olfactory dysfunction which was graded into anosmia, mild, moderate and severe microsmia 35% had either taste or smell loss 12% had only smell loss 7% had only taste loss 17% had combined smell and taste loss Of 83% patients who had anosmia/severe microsmia, only 35% was aware of their smell dysfunction.
| Objective assessment shows some degree of olfactory dysfunction and not always anosmia, is a major sign for COVID-19 Objective Olfactory testing may help in screening COVID-19 patients.
| This is the only study using objective testing for olfactory function using The University of Pennsylvania Smell Identification Test (UPSIT) Patients were graded based on severity Matched controls used
| Quantitative assessment not done for taste dysfunction. Included only hospitalised patients Smell recovery was not assessed
| Fair |
| 7 | Bénézit F et al., [27] April 2020 | 62% had hypogeusia 45% had hyposmia 43% had combined hyposmia and hypogeusia Hyposmia and hypogeusia were strongly associated with COVID-19 diagnosis independently and together
| These symptoms can be used for Mass screening and initial diagnosis of COVID-19
| Findings compared with COVID negative patients (with Influenza-Like Illness (ILI))
| No objective assessment No standard questionnaire Smell recovery was not assessed.
| Good |
| 8 | Vaira LA et al., [39] April 2020 | Smell: Olfactory threshold and odor discriminative ability assessed Taste: Standardised test to test four primary tastes 73.6% have one or more of olfactory-gustatory disturbance Most patients had mild-moderate olfactory and taste disorders (OTD) Older patients more prone to taste disturbance (p<0.003)
| OTD are common clinical findings in COVID-19 OTD occur in early stages of infection If sudden and OTD not associated with rhinitis symptoms, COVID-19 to be suspected
| Standardised objective tests used for taste and smell assessment Smell recovery was tested Studies relation of OTD with age and time of onset of clinical symptoms
| Evaluation of OTD performed at 19.3 days from the onset of clinical symptoms | Fair |
| 9 | Spinato G et al., [40] April 2020 | 64.4% reported one or more OTD 11.9% reported it as a presenting symptom 3% reported OTD as an isolated symptom STD more common in women
| Testing for COVID-19 and self-isolation to be considered in sudden onset of altered taste or smell
| Standard questionnaire- Sino-nasal outcome test 22 (SNOT-22) used Studied grades of OTD
| Smell and taste dysfunction not distinguished Objective testing not performed Only mildly symptomatic cases included
| Good |
| 10 | Lechein JR et al., [37] April, 20 | OTD more common in younger patients and in female patients Anomsia-70.2% Dysgeusia-54.2% of 1420 patients Anosmia persisted for 1 week in 37.5% of cured patients
| Smell disturbance is a key under estimated symptom of mild to moderated COVID-19 patients
| Multi-centre study with large sample size Varied ethnicity Age and sex association with COVID-19 assessed
| Only mild-moderate cases Young patients with few co-morbidities No objective testing
| Good |
| 11 | Yan CH et al., [17] April, 20 | Investigated association between smell loss and hospitalisation in COVID-19 Smell loss was strongly associated with COVID-19 which does not require hospitalisation (aOR 0.09 95% CI- 0.01-0.74) whereas positive findings on chest imaging was associated with hospitalisation
| Anosmia may be related to a mild disease, not requiring hospitalisation
| Included both mild and severe cases of COVID-19
| No objective testing Grades of smell dysfunction not assessed
| Good |
| 12 | Beltrán-Corbellini A et al., [30] April 20 | Investigate new onset olfactory and taste disorders (OTD) in COVID-19 compared to ILI (controls) 39.2% of cases had OTD compared to 12.5% in controls. Smell dysfunction-80% Taste dysfunction-90.3% Among COVID-19 patients, patients with OTD were younger than those without OTD Mean duration of OTD- 7.5+/- 3.2 days. 40% recovered completely after 1 week.
| OTD common in COVID-19 compared to Influenza-Like Illness (ILI) Usually it is a presenting symptom and acute in onset
| Case-control study Assessed recovery
| No objective testing
| Good |
| 13 | Tostmann A et al., [31] April 20 | Healthcare workers with mild symptoms tested for COVID-19 Anosmia reported in 47% COVID positive and strongly associated with COVID-positivity OR=23.0; 95% confidence interval (CI): 8.2-64.8) Predictive model based on these symptoms showed sensitivity: 91.2%; specificity: 55.6%
| Authors propose self-isolation to patients with olfactory symptoms
| Predictive model calculated
| No objective testing done Study subjects involved only those with mild symptoms
| Good |
| 14 | Wee LE et al., [32] April 20 | COVID testing done for all suspected cases including those with the symptoms of OTD 22.7% patients COVID positive subjects had OTD Odds ratio for OTD was higher for patients with COVID compared to those with other respiratory viruses (odds ratio, OR=10.14, 95% CI 2.37-43.49, p<0.001).
| OTD as a self-reported symptom had a high specificity (98.7%) as a screening tool in an Asian cohort
| Compared with COVID-negative cases with the same mild symptoms
| No objective testing performed
| Fair |
Qualitative synthesis: A total of 14 studies were included in the qualitative synthesis. The key findings, strength and limitations of various studies have been summarised in [Table/Fig-3].
Quantitative synthesis (Meta-analysis): Association between smell and taste disorders and COVID-19: Five studies compared the smell and taste function of patients with COVID-19 with those who were COVID-19 negative. Smell and taste function was assessed through questionnaires for four of these studies [27,30-32] whereas by objective testing for one study [23]. The pooled OR was 15.59 (95% CI, 6.31-38.55) [Table/Fig-4].
Association of olfactory and gustatory symptoms with COVID-19.
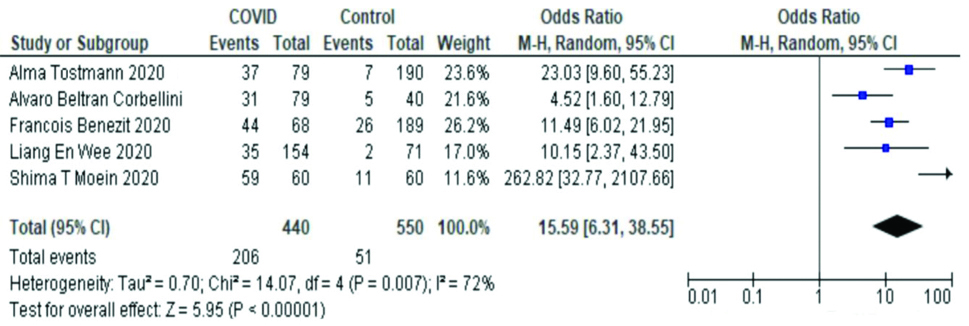
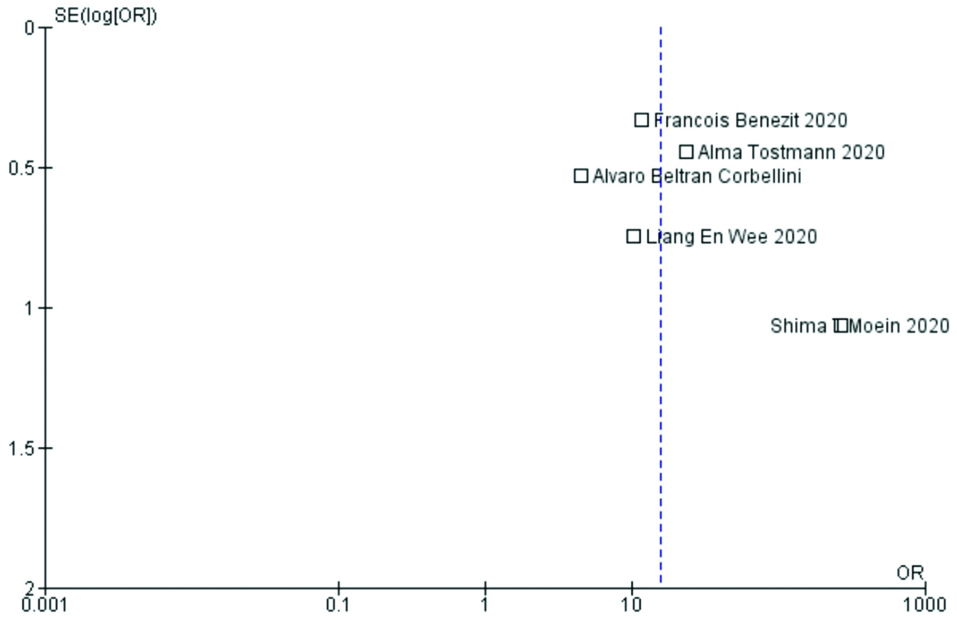
This reflects that smell and taste disorders are strongly associated with COVID-19. The studies had moderate heterogeneity by I2 test (72%). On the funnel plot, the study by Moein ST et al., appeared to be well away from the central line indicating a publication bias [Table/Fig-5] [23].
Funnel plot depicting publication bias.
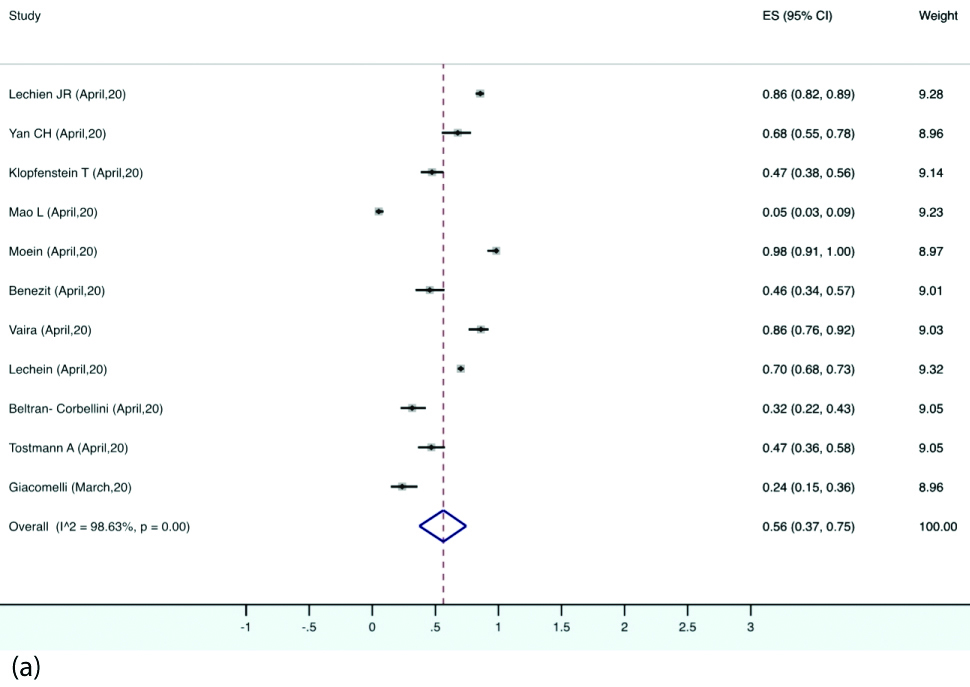
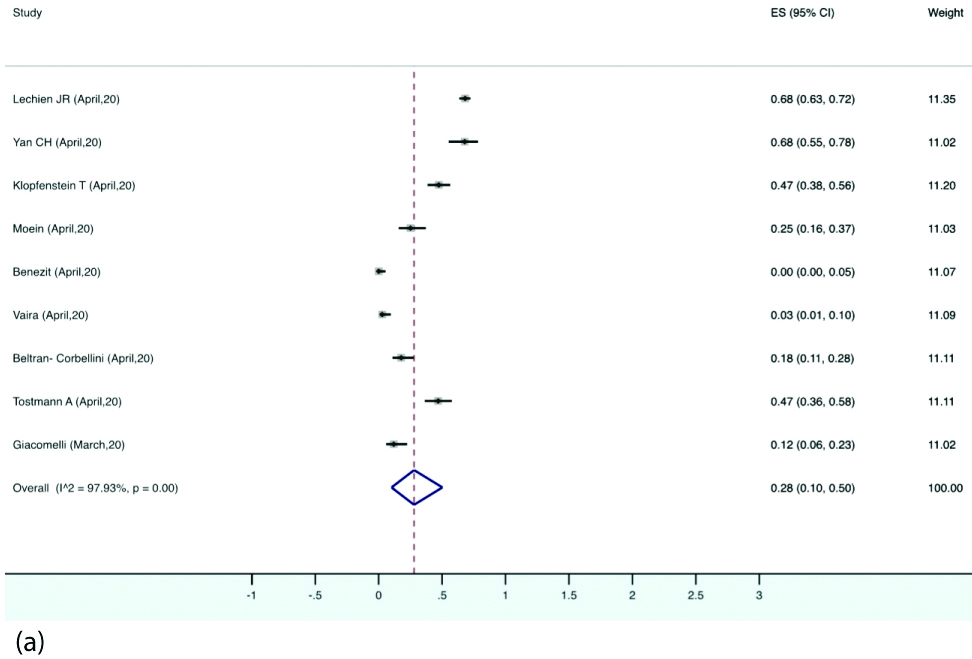
Prevalence of Olfactory and gustatory dysfunction: Olfactory dysfunction was reported by 12 studies. The pooled prevalence of olfactory dysfunction was 56% (95% CI 37-75) in the patients with COVID-19 [Table/Fig-6a]. The pooled prevalence for gustatory disturbance as reported by 11 studies was 44% (95% CI 28-62) [Table/Fig-6b]. A 32% of the patients reported both smell and taste disturbance (95% CI, 21-43) [Table/Fig-6c]. Nine studies reported data regarding OTD as presenting symptoms for which the prevalence was 9%. (95% CI, 3-17) [Table/Fig-6d]. Detailed statistical analyses are demonstrated in supplemental tables.
Forest plot illustrating pooled prevalence of olfactory dysfunction.
Forest plot illustrating pooled prevalence of gustatory dysfunction.
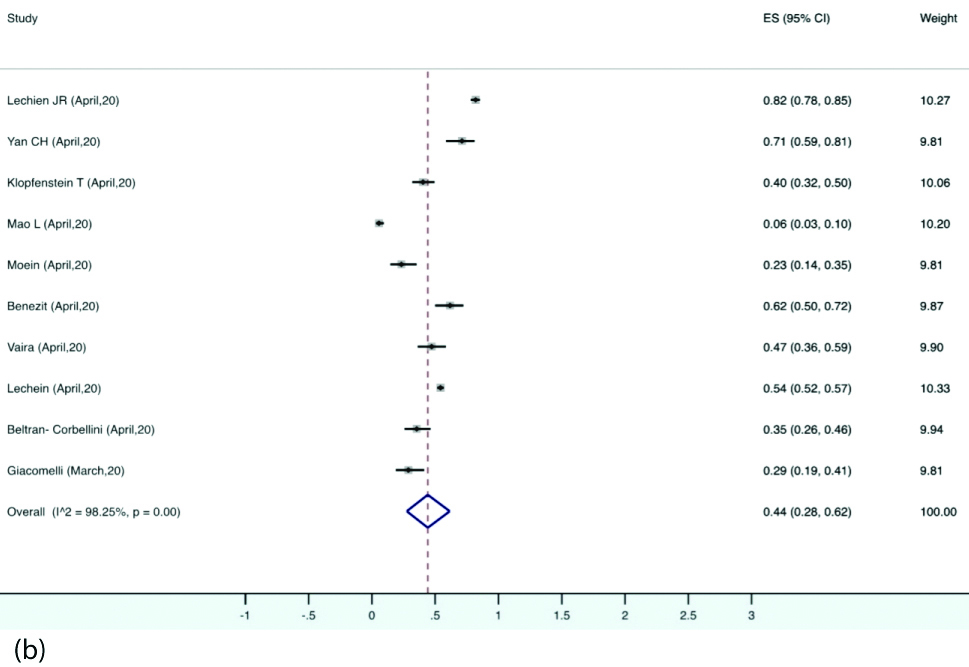
Forest plot illustrating pooled prevalence of both olfactory and gustatory dysfunction.
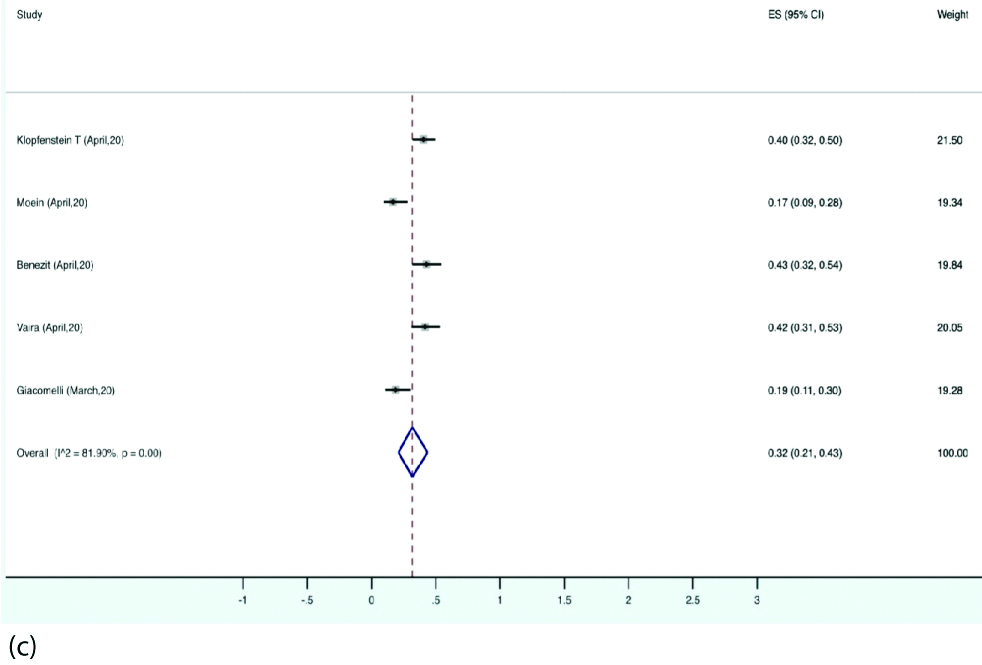
Forest plot illustrating pooled prevalence of olfactory and gustatory dysfunction as presenting symptoms.
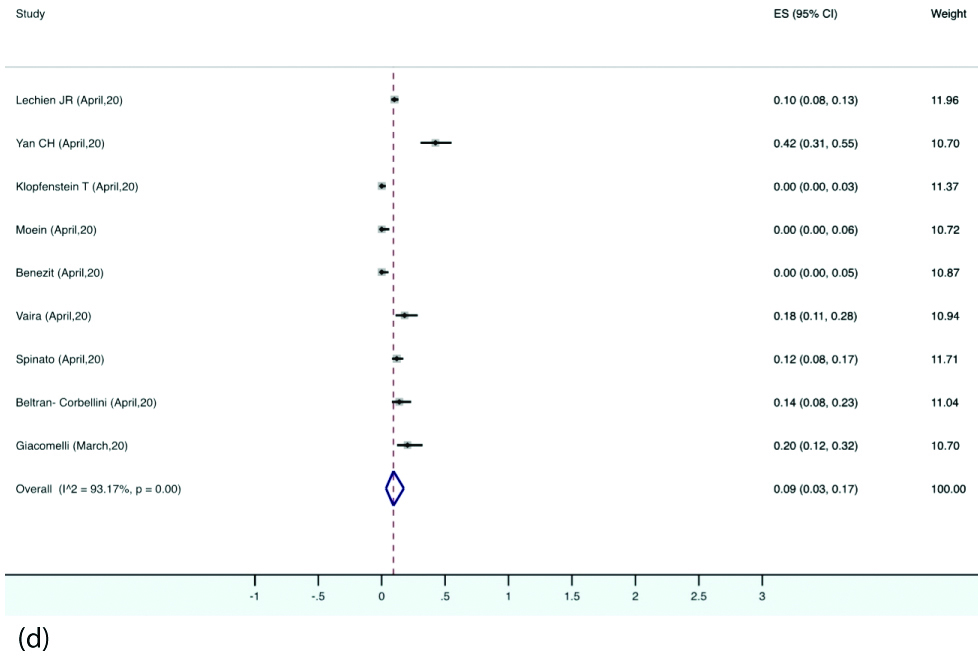
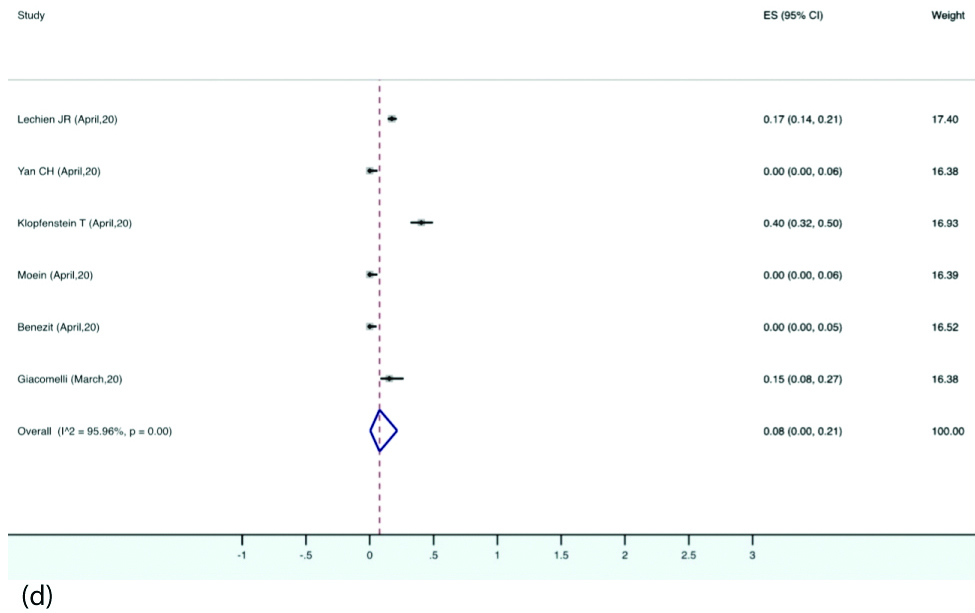
Prevalence of different types of smell and taste dysfunctions: The estimated pooled prevalence of anosmia was 28% (95% CI, 10-50) in the patients with COVID-19. Hyposmia was reported in eight studies, the pooled prevalence for which was 27% (95% CI, 6-54). Eight studies reported ageusia with estimated pooled prevalence of 12% (95% CI, 1-33). Dysgeusia was reported by six studies with pooled prevalence of 8% (95% CI, 0-21) [Table/Fig-7a,b,c and d].
Forest plot depicting pooled prevalence of anosmia.
Forest plot depicting pooled prevalence of hyposmia.
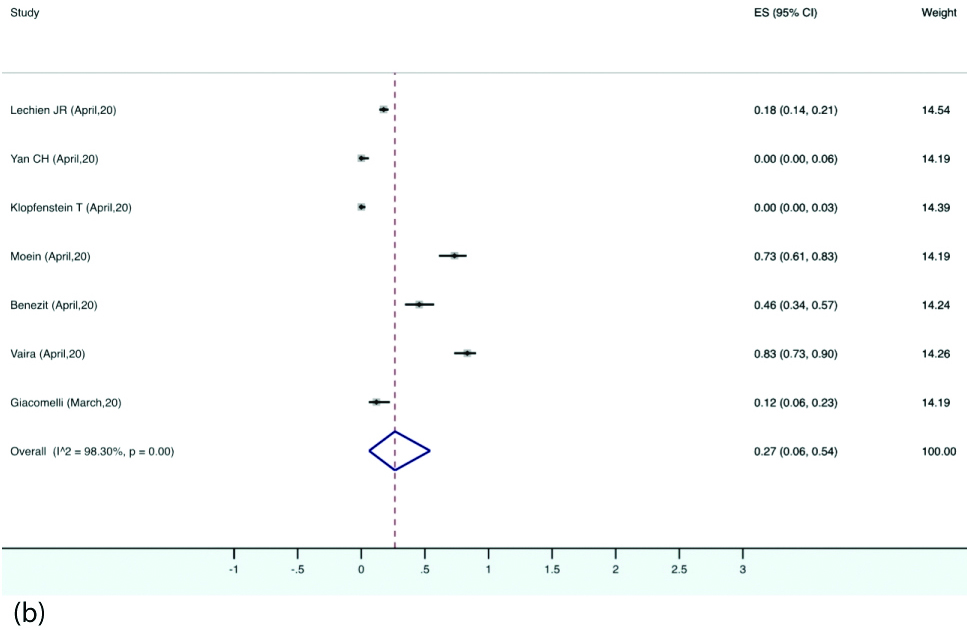
Forest plot depicting pooled prevalence of ageusia.
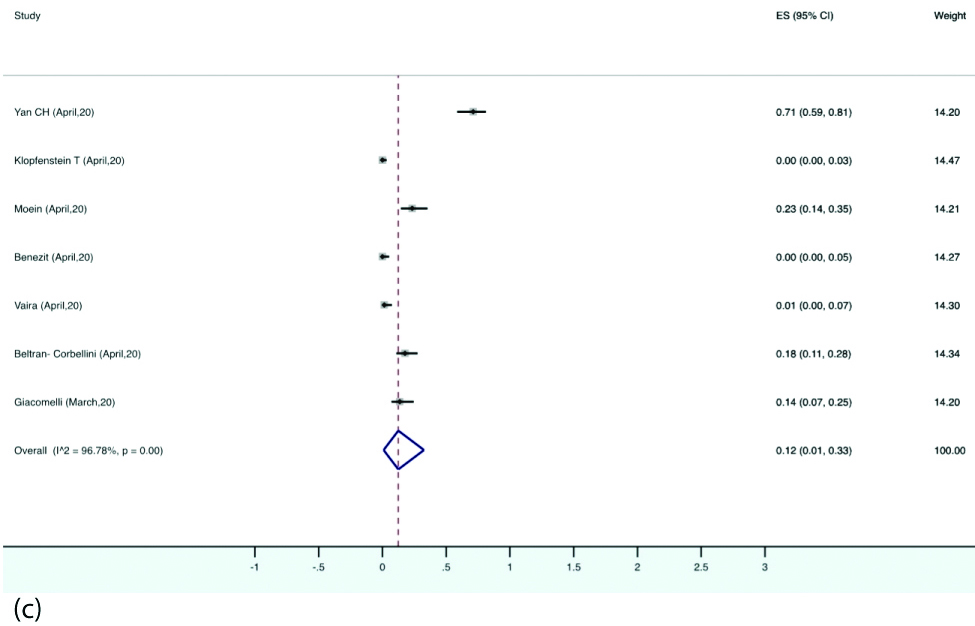
Forest plot depicting pooled prevalence of dysgeusia.
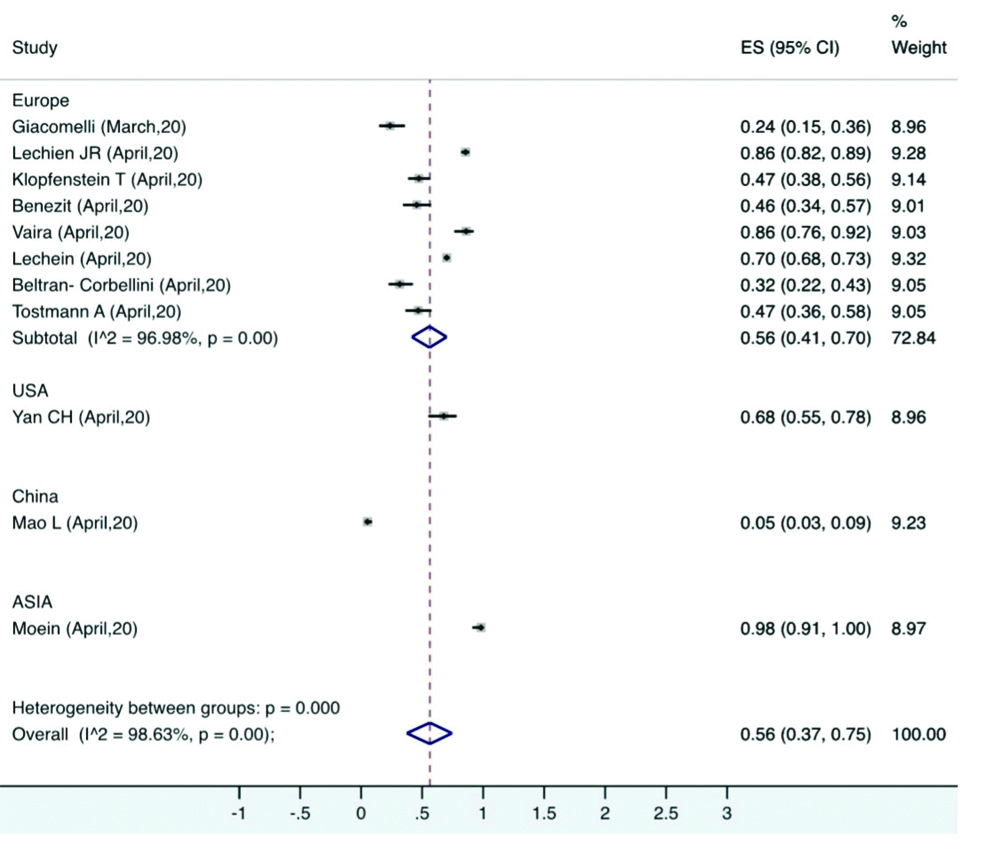
Subgroup analysis: A high degree of heterogeneity was found across various studies. Studies were performed in different countries with wide ethnicity. A region-wise subgroup analysis was performed for olfactory and gustatory dysfunction. The prevalence ranged from 5% (China) to 98% (Asia). Pooled prevalence involving only the European countries was 56%, which was similar to the overall pooled prevalence [Table/Fig-8].
Subgroup analysis for prevalence of olfactory dysfunction based on geographical location.
Discussion
There is growing anecdotal evidence of sudden anosmia or hyposmia with or without other symptoms of COVID-19 infection [10]. This systematic review and meta-analysis involving 13 studies and 3,125 patients demonstrates a significant association between olfactory and gustatory dysfunction and COVID-19. It also demonstrates a high prevalence of these symptoms in patients with COVID-19.
Pooled OR across the five case-control studies was 15.59 showing that OTD were 15 times more common in patients with COVID-19 compared to those without. Lechien JR et al., found olfactory and gustatory symptoms show a significant correlation with each other [6]. Yan CH et al., showed that among all the symptoms, anosmia and ageusia displayed the strongest association with COVID-19 positivity and these symptoms were 10 times more common than those in COVID-19 negative individuals [17]. This reflects the importance of adding anosmia and ageusia as important components of screening symptoms for COVID-19.
The pooled prevalence of olfactory dysfunction was 56% while that of anosmia and hyposmia was 28% and 27%, respectively [6,16,17,22,27]. It is noteworthy that smell dysfunction in the studies ranged from as low as 5% (Mao L et al.,) to 98% (Moein ST et al.,) with the other authors reporting a smell dysfunction of 23.7%, 85.6%, 67.8%, 47% and 45% [6,16,17,21-23]. Mao L et al., (smell dysfunction-5%) have based their findings on electronic medical records which could have led to under-reporting of minor symptoms such as that related to olfaction [21]. Recent evidence suggests that Angiotensin Converting Enzyme 2 (ACE-2) receptor to which coronavirus binds, might have some diversity across the populations. This may in turn lead to its variable association with SARS-CoV-2. This difference may translate into the difference in symptoms and clinical outcomes of patients that is found among different ethnicities [6,41]. It is noteworthy that in COVID-19 disease, olfactory symptoms were seen without nasal obstruction or rhinorrhoea unlike other viral infections [42]. The aetiology of olfactory and gustatory symptoms in COVID-19 is elusive and various theories have been proposed for the same [32]. One of the proposed aetiology is the possible neuroinvasive potential of SARS-CoV-2 [43]. Trans-neural penetration of the SARS-CoV virus into the central nervous system through the olfactory bulb has been demonstrated in a mice model [44]. Significant neuronal death has been observed in brain due to rapid spread of SARS-CoV [6]. A similar neuroinvasive potential of the SARS-CoV-2 may have a major role not only in causing OTD but also in causing respiratory failure of patients with COVID-19 [45]. Another proposed mechanism is due to the binding of SARS-CoV-2 to Angiotensin converting enzyme 2 (ACE-2) receptors. Since the taste buds and olfactory epithelium also express ACE-2, SARS-CoV-2 may have a cytopathic effect resulting in these symptoms.
This meta-analysis found gustatory disturbance of 44% in COVID-19 patients. Some studies also reported the type of taste dysfunction as ageusia and dysgeusia, the pooled prevalence of which were 12% and 8%, respectively. In the systematic review, most of the studies reported gustatory dysfunction either in combination with smell dysfunction or in isolation [6,16,17,21-23,30,37,39]. Except for the basic taste types of sour, sweet, salty, umami and bitter, the rest of the gustatory flavours are attributed to the olfactory system. Thus in many cases, taste loss may be attributed to a loss of smell rather than an actual damage to the taste buds [23,46,47].
OTD was reported as presenting symptom with an estimated pooled prevalence of 9% across nine studies [6,16,17,22,23,27,30,39,40]. Since these symptoms have not been identified as screening symptoms by major health organisations, they may often get missed leading to underdiagnosis of COVID-19. Giacomelli A et al., noted taste alteration as an initial symptom before hospitalisation in 91% of the patients [16]. Moreover, a few studies also reported sudden onset anosmia with or without dysgeusia as an isolated symptom of COVID-19 in otherwise asymptomatic patients [19,24,48].
Olfactory disturbances showed a gender predilection towards females across a number of studies [6,16,17]. with the values being statistically significant in the study done by Lechien JR et al., and Giacomelli A et al., [6,16]. Lechien JR et al., have suggested that this could be due to a possible heightened inflammatory response in females compared to males, warranting further studies to investigate the gender susceptibility to anosmia [6,35].
Many studies assessed smell recovery within a time frame of one week to four weeks. Most studies reported that the smell loss was transient, with a variable recovery after two weeks [6,17,22]. Lechien JR et al., pointed out that smell recovery takes a longer time compared to the recovery of other symptoms [6]. The authors observed a wide amount of heterogeneity in the meta-analysis which is a common finding in most of the studies pertaining to COVID-19 [49]. A subgroup analysis was done based on the prevalence of these symptoms across different countries.
Limitation(s)
This systematic review has various limitations considering the paucity of data available related to olfactory and taste disturbances in COVID-19. Most of the studies included were cross-sectional studies, where the data was collected either retrospectively from medical records or through a survey, without a standard follow-up [16,21,22,27]. The data was mostly collected in the form of a questionnaire except for two studies by Moein ST et al., and Vaira LA et al., which involved an objective testing method [23,39]. Of the studies involving questionnaires, only Lechein JR et al., and Spinato G et al., used standard validated questionnaires [6,40]. Most of the data was collected through internet and phone calls, questioning its reliability. Moein ST et al., reported that of the 85% patients that reported ansomia or severe microsmia, only 35% were aware of their smell dysfunction [23]. From this, it can be inferred that a sizeable population may be unaware of their olfactory disturbances leading to a recall bias in questionnaire-based studies.
Conclusion(s)
Since most of the studies were based on self-reported symptoms, more studies with validated objective testing of olfactory and gustatory disturbances in larger sample size are required. It can be concluded that there is a significant association between olfactory and gustatory symptoms and COVID-19 disease. Majority of the studies support the use of these symptoms as screening tools for COVID-19. This could help in early diagnosis and to contain disease transmission, especially from otherwise asymptomatic cases. Considering the ongoing exponential spread of SARS-CoV-2, limited diagnostic tests and scarce health resources worldwide, the authors emphasise the use of olfactory and taste disturbance as early markers of the disease.
SD: Standard deviation