GBC is considered as a relatively rare neoplasm worldwide with poor prognosis, rising incidence in the recent past, and unfortunately less treatment options. It occupies the sixth most common cancer of Gastro Intestinal Tract (GIT) and forms 80-95% of biliary tract carcinomas. It is predominantly a disease of elderly female; it affects women two to six times more than men [1].
GBC is usually diagnosed at an advanced state, therefore it is characterised by high mortality with an overall survival rate of six months [2]. It has a wide geographical variation that may be due to various risk factors, which include cholelithiasis (especially untreated chronic calculous cholecystitis), obesity, and reproductive factors. These suspected factors likely represent promoters of carcinogenesis [3]. The molecular biology of GBC is unclearly understood. Most of these studies have focused on the gene abnormalities and deletion of R as, TP53, and p16Ink4/CDKN2 [4].
Tyrosine Kinase (TK) has been recently implicated in pathogenesis of various neoplasms. Several oncogenes, which encode for growth factor receptors, have TK activity. The EGFR family includes the Epidermal Growth Factor Receptor (EGFR, HER-1) and c-erbB-2 (HER2) are with TK activity [5]. HER2 is a normal cellular gene, also called c-erbB2, located on chromosome 17q12q21.28. It encodes a TK that is strongly related to receptor for EGFR [6]. The overexpression of this receptor due to gene amplification and protein overexpression is widely detected in various solid tumours [7].
Mucins (known as high molecular weight glycoproteins) plays vital roles in carcinogenesis or tumour invasion. There are about 20 MUC-encoding genes, with cell membrane localisation (MUC2, MUC5AC, MUC6) and transmembranous mucins (MUC1, MUC4) [8]. Normally, MUC1 expression is present in breast, pancreas, the respiratory tract, salivary gland, and prostate, but sparse in GIT including the gall bladder. It is a high molecular weight transmembrane glycoprotein that is involved in lubrication and hydration of luminal surface. In the pathogenesis of various cancers, MUC1 has an important role in cell adhesions and cellular interactions [9].
In the recent times, these oncogenes were widely studied and were evaluated with the availability of specific chemotherapeutic targets against them in cancer treatment plan. Only few studies focusing on expression of HER2/neu and MUC1 in GBC exist. Ki-67 is an important proliferative marker, which is expressed in all phases of the cell cycle except G0. It corresponds to a nuclear non-histone protein which was described in 1983 [10]. It can be exclusively detected within the nucleus of proliferating tumour cells, so the fraction of Ki-67-positive tumour cells (the Ki-67 LI) is considered, as an excellent prognostic marker. It is associated with ribosomal RNA transcription. Ribosomal RNA synthesis is inhibited when antigen Ki-67 is inactivated [11].
The objective of this study was aimed to investigate the expression of HER2/ neu, MUC1 and Ki-67 in GBC, comparing with premalignant lesions and cholecystitis as control by immunohistochemistry, to elucidate their relation to the carcinoma development and to correlate them with different clinicopathological parameters of GBC as prognostic markers.
Materials and Methods
This retrospective study was conducted in Department of Pathology of Banha University Hospital, January 2012 to December 2019. The study included 83 blocks and related clinical data. Cases were classified into four groups:
Malignant group: GBC (40 cases)
Premalignant group: dysplasia (8 cases)
Metaplastic group: chronic cholecystitis with intestinal and pseudo pylori metaplasia (10 cases)
Chronic cholecystitis without metaplastic and dysplastic changes, as a control group (25 cases)
Archived specimens were retrieved from archived records in hospital information system. Sections were prepared at four micron thickness from each tissue block, one of them was stained by Haematoxylin and Eosin (H&E) for histopathological re-evaluation. The sections were reviewed and graded according to World Health Organisation (WHO) classification and 8th edition of AJCC staging system (2019) [12].
Immunohistochemical staining: Sections were pretreated by sodium citrated buffer (pH-6) as heat-antigen retrieval for 15 minutes in microwave. The sections were then cooled for five minutes and rinsed in tapwater. After blockage of biotin and peroxidase, immunohistochemical staining was performed and left for incubation period as shown in [Table/Fig-1]. Slides were subsequently stained by the universal immunoperoxidase method, according to the manufacturer’s protocol. All study cases were approved by Ethical Committee in Benha Faculty of Medicine (Rc2.10.2020).
| Antibody | Source | Dilution | Incubation period | Positive control |
|---|
| HER2/neu | LAB vision | Ready to use | 60 min | Breast carcinoma |
| MUC1 | Novocastra) | Ready to use | 30 min | Lung tissue |
| KI-67 | Thermo scientific | Ready to use | 60 min | Breast carcinoma |
HER2/neu expression interpretation: CAP/ASCO (College of American Pathologists/American Society of Clinical Oncology) guidelines were used to measure HER2/neu positivity as in [Table/Fig-2]. The immunohistochemical criteria for HER2/neu positivity in breast cancer was applied [13].
| Score | Criteria |
|---|
| 0 (negative) | No immunoreactivity or immunoreactivity in 10% of tumour cells but only a portion of the membrane is positive (Incomplete). |
| +1 (negative | Faint weak immunoreactivity in >10% of tumour cells but only a portion of the membrane is positive (Incomplete). |
| +2 (positive) | Weak to moderate complete membrane immunoreactivity in >10% of tumour cells. |
| +3 (positive) | Moderate to strong complete immune reactivity in >10% of tumour cells. |
MUC1 interpretation: The presence of MUC1 expression was graded based on the sum score of expression rate and intensity of staining [Table/Fig-3] [14].
MUC1 immunostaining score [14].
| Score | Criteria |
|---|
| 0 (negative) | Percentage of positive cells in <10% |
| +1 | Percentage of positive cells in 10-25% |
| +2 | Percentage of positive cells in 26-50% |
| +3 | Percentage of positive cells in >50% |
The positive intensity was divided into two groups: 1 (faint) and 2 (strong) for statistical analysis, sum scores (0-5) of the extent and intensity scales were divided into low expression (sum score 0-3) and high expression (sum score 4-5).
Ki-67 expression interpretation: Ki-67 LI was calculated as the percentage of positively stained tumour cell nuclei out of the total tumour cells counted (n=1000). A percentage of stained cells was considered positive regardless of the intensity of staining. A percentage ≥20% of stained cells was considered positive, regardless of the intensity of staining [15].
Statistical Analysis
The data collected were analysed using SPSS version 16.0. Determining the probability factor (p-value) assessed the significance of results. Chi-square test or Fisher’s-exact tests were applied to evaluate the relation between variables. The p-value <0.05, the results were considered statistically significant.
Results
This retrospective study included four groups, classifying into: 1) malignant group of 40 gall bladder adenocarcinoma cases with the median age of this group as 53.6±12.46 years (ranged from 39 to 79 years) and female predominance (80%). 2) Eight cases of dysplasia, 3) 10 cases of metaplastic changes (antral and intestinal) 4) finally 25 cases of cholecystitis taken as control group with the median age of studied 25 cholecystitis cases as 39.4±5.7 years (ranged from 28 to 70 years) and female predominance (90%).
Immunohistochemical results: Positive HER2/neu expression (+2, +3) was detected in 47.5% (19/40) of malignant cases and 12.5% (1/8) of dysplastic group was positive, at the same time it was completely absent in the metaplastic group and control cases (p<0.01). High MUC1 expression was found in 75% of both malignant (30/40), and dysplastic (6/8) studied cases beside 10% (1/10) of metaplastic group p<0.01. All cases of malignant group 100% (40/40), 50% (4/8) of dysplastic, 10% of metaplastic (1/10) and none of control group (0/25) revealed cytoplasmic MUC1 expression p<0.01. Ki-67 positive expression was registered in 55% (22/40) of malignant group while it was completely absent in the other three studied groups [Table/Fig-4].
HER2/neu, Ki-67 and MUC1 expression in studied four groups.
| Variables | Malignant (n=40) | Premalignant (n=8) | Metaplastic (n=10) | Cholecystitis (n=25) | p-value |
|---|
| HER2/neu | Negative | 21 (52.5%) | 7 (87.5%) | 10 (100%) | 25 (100%) | <0.01** |
| Positive | 19 (47.5%) | 1 (12.5%) | 0 (0%) | 0 (0%) |
| MUC1 | Scoring | Low | 10 (25%) | 2 (25%) | 9 (90 %) | 25 (100%) | <0.01** |
| High | 30 (75%) | 6 (75%) | 1 (10%) | 0 (0%) |
| Pattern | Apical | 0 (0%) | 4 (50%) | 9 (90 %) | 25 (100%) | <0.01** |
| Cytoplasmic | 40 (100%) | 4 (50%) | 1 (10%) | 0 (0%) |
| Ki-67 | <20% | 18 (45%) | 8 (100%) | 10 (100%) | 25 (100%) | <0.01** |
| ≥20% | 22 (55%) | 0 (0%) | 0 (0%) | 0 (0%) |
**significant
Correlation between HER2/neu and clinicopathological factors in carcinoma group: HER2/neu overexpression was more evident in older patients, 50 years of age (p=0.001, r=0.696), site of the tumour (p=0.001) (r=0.441). HER2/neu overexpression did not correlate significantly with sex, level of tumour infiltration (T), presence of lymph node or distant metastasis. There was no significant difference in expression between early and advanced stages [Table/Fig-5, 6 and 7].
HER2/neu scoring according to clinicopathological variables of malignant group.
| Variable | Total | HER2/neu | p-value |
|---|
| Negative n (%) | +1 n (%) | +2 n (%) | +3 n (%) |
|---|
| Age (years) |
| <50 | 17 | 12 (70.6%) | 3 (17.6%) | 2 (11.8%) | 0 | 0.001** |
| ≥50 | 23 | 2 (8.7%) | 4 (17.4%) | 6 (26.1%) | 11 (47.8%) |
| Mean±SD | 53.6±12.46 |
| Sex |
| Female | 26 | 7 (26.9%) | 4 (15.4%) | 6 (23.1%) | 9 (34.6%) | 0.076 |
| Male | 14 | 7 (50%) | 3 (21.4%) | 2 (14.3%) | 2 (14.3%) |
| Site |
| Fundus | 16 | 12 (75%) | 1 (6.2%) | 1 (6.2%) | 2 (12.5%) | 0.001** |
| Body | 13 | 1 (7.7%) | 4 (30.8%) | 4 (30.8%) | 4 (30.8%) |
| Neck | 11 | 1 (9.1%) | 2 (18.2%) | 3 (27.3%) | 5 (45.5%) |
| Grade |
| WDC | 9 | 2 (22.2%) | 1 (11.1%) | 1 (11.1%) | 5 (55.6%) | 0.532 |
| MDC | 20 | 10 (50%) | 4 (20%) | 4 (20%) | 2 (10%) |
| PDC | 11 | 2 (18.2%) | 2 (18.2%) | 3 (27.3%) | 4 (36.3%) |
| Infiltration level |
| T1 | 10 | 6 (60%) | 2 (20%) | 1 (10%) | 1 (10%) | 0.293 |
| T2 | 17 | 6 (35.5%) | 1 (5.9%) | 4 (23.5%) | 6 (35.3%) |
| T3 | 13 | 2 (15.4%) | 4 (30.8%) | 3 (23.1%) | 4 (30.8%) |
| LN |
| Negative | 19 | 6 (31.6%) | 2 (10.5%) | 5 (26.3%) | 6 (31.6%) | 0.391 |
| Positive | 21 | 8 (38.1%) | 5 (23.8%) | 3 (14.3%) | 5 (23.8%) |
| DM |
| Absent | 24 | 11 (45.8%) | 4 (16.7%) | 5 (20.8%) | 4 (16.7%) | 0.720 |
| Present | 16 | 3 (18.7%) | 3 (18.8%) | 3 (18.8%) | 7 (43.7%) |
| Stage |
| I | 10 | 6 (60%) | 2 (20%) | 1 (10%) | 1 (10%) | 0.775 |
| II | 8 | 1 (12.5% | 2 (25%) | 3 (37.5 | 2 (25%) |
| III | 6 | 2 (33.3%) | 2 (33.3%) | 1 (16.7%) | 1 (16.7%) |
| IV | 16 | 5 (31.2%) | 1 (6.2%) | 3 (18.7%) | 7 (43.9%) |
| Total | 40 | 14 (35%) | 7 (17.5%) | 8 (20%) | 11 (27.5%) | |
WDC: Well differentiated carcinoma; MDC: Moderately Differentiated Carcinoma; PDC: Poorly differentiated carcinoma; LN: Lymph node; DM: Distant metastasis; **significant
HER2/neu expression in GBC showing moderate to strong complete membranous immunoreactivity in >10% of tumour cells score 3 (Avidin Biotin Complex ABC x400).
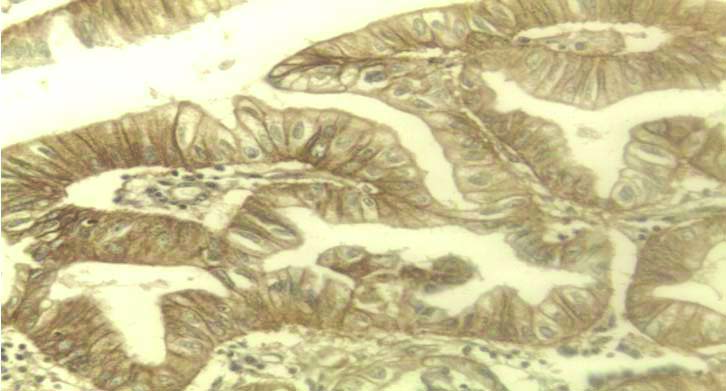
HER2/neu expression in dysplastic gland showing weak to moderate complete membrane immunoreactivity in >10% of tumour cells score 2 (Avidin Biotin Complex ABC x400).
Correlation between Ki-67 and clinicopathological factors in malignant group: Ki-67 positive expression was found in 55% (22/40) of studied malignant cases. Ki-67 LI significantly correlated with age (p=0.001) (r=0.493), and stage of the tumour (p=0.012) (r=0.433), while other variables showed no significant difference, despite its expression was more in positive LN metastatic cases than in cases with no LN metastasis, as shown in [Table/Fig-8,9a-b].
Correlation between Ki-67 and clinicopathological factors in carcinoma.
| Variable | Total | Ki -67 labeling index (LI) | p-value |
|---|
| LI <20% n (%) | LI ≥20% n (%) |
|---|
| Age (years) |
| <50 | 17 | 1 (5.9%) | 16 (94.1%) | 0.001** |
| ≥50 | 23 | 17 (73.9%) | 6 (26.1%) |
| Sex |
| Female | 26 | 10 (38.5%) | 16 (61.5%) | 0.603 |
| Male | 14 | 8 (57.2%) | 6 (42.8%) |
| Site |
| Fundus | 16 | 6 (37.5%) | 10 (62.5%) | 0.231 |
| Body | 13 | 7 (53.8%) | 6 (46.2%) |
| Neck | 11 | 5 (45.5%) | 6 (54.5%) |
| Grade |
| WDC | 9 | 5 (55.6%) | 4 (44.4%) | 0.105 |
| MDC | 20 | 8 (40%) | 12 (60%) |
| PDC | 11 | 5 (45.5%) | 6 (54.5%) |
| Infiltration level |
| T1 | 10 | 7 (70%) | 3 (30%) | 0.640 |
| T2 | 17 | 7 (41.2%) | 10 (58.8%) |
| T3 | 13 | 4 (30.8%) | 9 (69.2%) |
| LN |
| Negative | 19 | 9 (47.7%) | 10 (52.3%) | 0.241 |
| Positive | 21 | 9 (42.9%) | 12 (57.1%) |
| Metastasis |
| Absent | 24 | 11 (45.8%) | 13 (54.2%) | 0.756 |
| Present | 16 | 7 (43.7%) | 9 (56.3%) |
| Stage |
| I | 10 | 8 (80%) | 2 (20%) | 0.012* |
| II | 8 | 4 (50%) | 4 (50%) |
| III | 6 | 2 (33.3%) | 4 (66.7%) |
| IV | 16 | 4 (25%) | 12 (75%) |
| Total | 40 | 18 (45%) | 22 (55%) | |
WDC: Well differentiated carcinoma; PDC: Poorly differentiated carcinoma; LN: Lymph node; DM: Distant metastasis
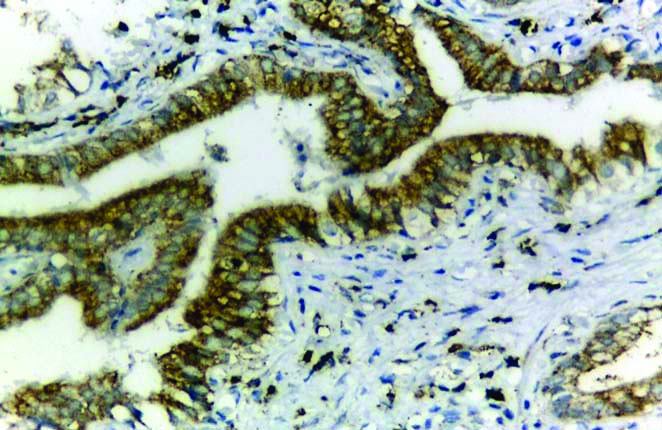
a) High nuclear Ki- 67expression in GBC (Avidin Biotin Complex (ABC) 400X; b) Negative Ki-67 expression in dysplastic glands (Avidin Biotin Complex (ABC) x200.
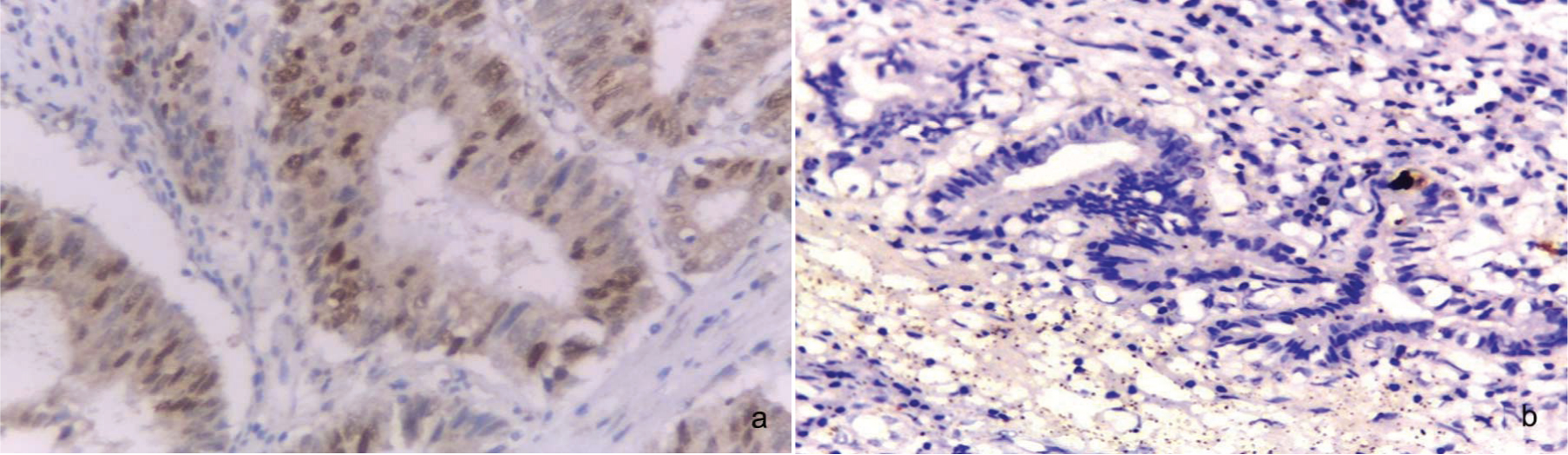
Correlation between MUC1 extension rate and clinicopathological factors in carcinoma: There was a significant difference in MUC1 expression among age group (p=0.0001) (r=0.591), sex of the patient (p=0.001) (r=0.498), grade groups (p=0.001) (r=0.503), tumour site (p=0.002) (r=0.485), level of infiltration (p<0.01) (r=0.493), the presence of lymph node (p=0.001) (r=0.516), distant metastasis (p=0.027) (r=0.349), and tumour stage (p=0.01) (r=0.526), as detailed in [Table/Fig-10,11a-b,12].
Correlation between MUC1 expression and clinicopathological factors in carcinoma.
| Variable | Total | MUC1 | p-value |
|---|
| Low n (%) | High n (%) |
|---|
| Age |
| <50 | 17 | 11 (64.7%) | 6 (35.3%) | 0.0001** |
| ≥50 | 23 | 2 (8.7%) | 21 (91.3%) |
| Sex |
| Female | 26 | 4 (15.4%) | 22 (84.6%) | 0. 001** |
| Male | 14 | 9 (64.3%) | 5 (35.7%) |
| Site |
| Fundus | 16 | 9 (65.2%) | 7 (43.8%) | 0. 002* |
| Body | 13 | 4 (30.8%) | 9 (69.2%) |
| Neck | 11 | 0 | 11 (100%) |
| Grade |
| WDC | 9 | 7 (77.8%) | 2 (22.2%) | 0. 001** |
| MDC | 20 | 5 (25%) | 15 (75%) |
| PDC | 11 | 1 (9.1%) | 10 (90.9%) |
| Infiltration level |
| T1 | 10 | 8 (80%) | 2 (20%) | 0.001** |
| T2 | 17 | 3 (16.7%) | 14 (82.4%) |
| T3 | 13 | 2 (15.4%) | 11 (84.6%) |
| LN |
| Negative | 19 | 11 (57.9%) | 8 (42.1%) | 0.001** |
| Positive | 21 | 2 (9.5%) | 19 (90.5%) |
| Metastasis |
| Absent | 24 | 11 (45.8%) | 13 (54.2%) | 0. 027* |
| Present | 16 | 2 (12.5%) | 14 (87.5%) |
| Stage |
| I | 10 | 8 (80%) | 2 (20%) | 0.01** |
| II | 8 | 2 (25%) | 6 (75%) |
| III | 6 | 1 (16.7%) | 5 (83.3%) |
| IV | 16 | 2 (12.5%) | 14 (67.5%) |
| Total | 40 | 13 (32.5%) | 27 (67.5%) |
WDC: Well differentiated carcinoma; MDC: Moderately differentiated carcinoma; PDC: Poorly differentiated carcinoma; LN: Lymph node; DM: Distant metastasis; **Significant
a) Apical membranous and low MUC1 expression in cholecystitis (Avidin Biotin Complex (ABC) X100; b) Apical membranous and cytoplasmic MUC1 expression in dysplasia (Avidin Biotin Complex (ABC) X200.
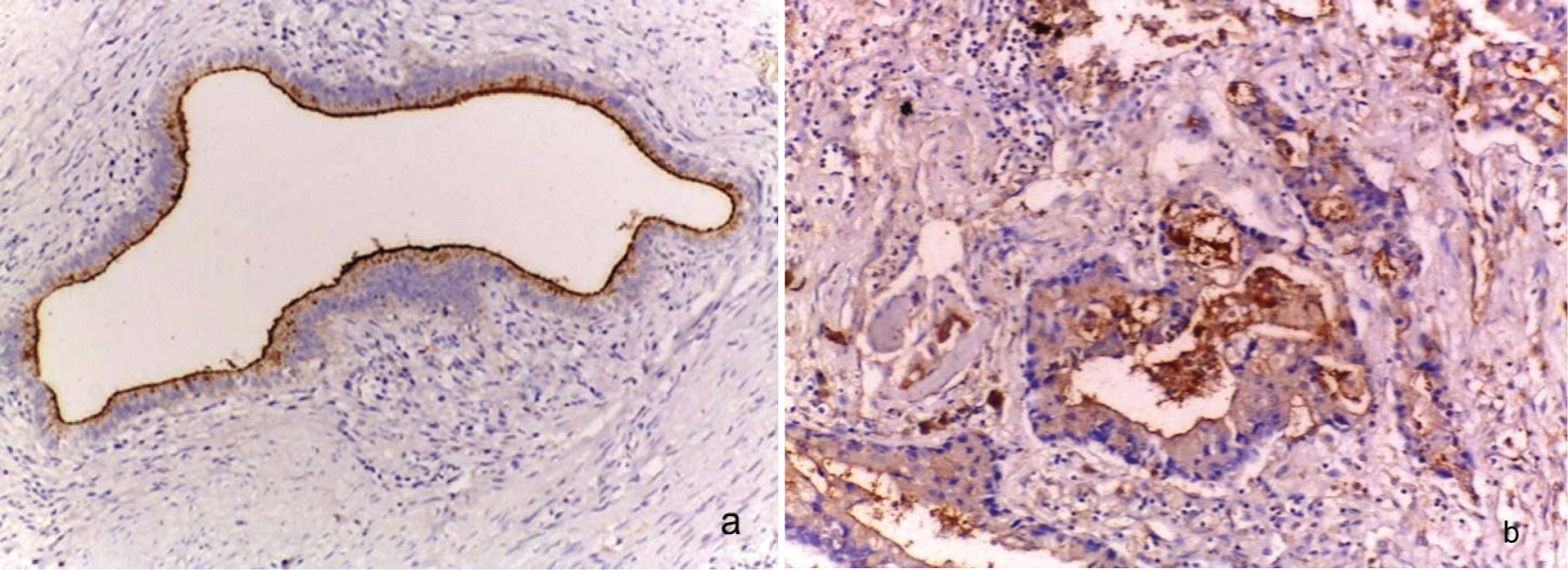
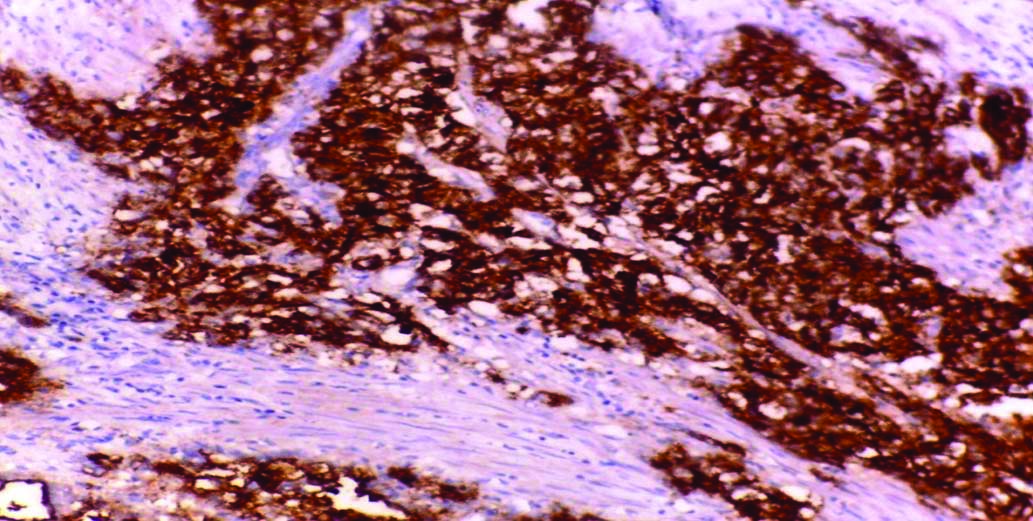
High cytoplasmic expression of MUC1 expression in GBC (Avidin Biotin Complex ABC x400).
Correlation between HER2/neu expression, MUC1 and Ki-67: There was an insignificant correlation between HER2/neu and Ki-67 expression within carcinoma cases (p=0.28) (r=0.667). Among 22 cases that were positive for Ki-67, 10 cases (45.5%) registered positive HER2/neu expression. Although most of the HER2/neu positive cases (55.5%) (15/27) showed high MUC1 expression but with no statistical correlation (p=0.501) as shown in [Table/Fig-13].
Relation between HER2/neu, Ki-67 and MUC1 expression within malignant group.
| Total | HER2/neu negative | HER2/neu positive | p-value |
|---|
| Ki-67 | <20% | 18 | 9 (50%) | 9 (50%) | 0.28 |
| ≥20% | 22 | 12 (54.5%) | 10 (45.5%) |
| MUC1 | Low | 13 | 9 (69.2%) | 4 (30.8%) | 0.501 |
| High | 27 | 12 (44.5%) | 15 (55.5%) |
Discussion
Carcinoma of gall bladder, an aggressive disease with poor prognosis, is the most common malignancy of the biliary tract and the sixth most common malignancy of the GIT worldwide. The poor prognosis, increasing incidence besides ineffective therapy of GBC make its management challenging. Therefore, there is a need of effective therapeutic agents for proper targeted therapy [16].
The present study was planned to investigate the expression of HER2/neu, MUC1and Ki-67 in GBC, comparing with dysplastic and metaplastic lesions, beside the correlation with clinicopathological parameters of GBC by immunohistochemical staining technique. Subsequently, these markers were studied to evaluate their value as targeted biomarkers in the therapy of GBC treatment.
The mean age of patients in the malignant group was 53.6±12.46 years, which was higher than that in control group 39.4±5.7 years. Female predominance was noted. This observation is comparable to that of Chaube A et al., [17]. HER2/neu, a member of the EGFR family, acts as an oncogene. It plays pivotal role in tumourigenesis of breast cancer. Its overexpression, as a result of gene amplification, is reported in other solid tumours [18].
In this current study, 47.5% (19/40) of GBC cases, and 12.5% (1/8) of dysplastic group, respectively were HER2/neu positive compared to the control group (cholecystitis) and metaplastic group in which HER2/neu expression was completely absent.
Positive expression of HER2/neu in GBC ranges from 2-46.5% across many studies [19-21]. This wide range can be due to variable methodology and different HER2/neu scorning system in these different studies. Some authors [17,22] considered +2 and +3 score as positive, as done in present study while Roa I et al., considered only +3 to be HER2 /neu positive [23]. Both of Doval DC et al., [24] and Pujani M et al., [25] used breast cancer scoring system for HER2/neu. This wide controversy highlights the need for a uniform consensus for HER2/neu scoring in GBC like that in breast carcinoma.
Close to present study results, HER2/neu was registered in 24% of GBC cases in study done by Pujani M et al., and absence of its expression in control group [25]. The study done by Yoshida H et al., supports present study findings, as HER2/neu positive expression was in 23% of GBC cases [26]. In the same study, all non-neoplastic gall bladder lesions were HER2/neu negative. The results of Roa I et al., who registered HER2/neu overexpression in GBC with negative immune-staining in the control group match with the present study [23].
This is in contrast with findings, reported by Zhou YM et al., who registered that 70.7% of GBC with positive expression of HER2/neu and it was higher in dysplasia lesions (85.5%) [27].
Although HER2/neu score (+2) and (+3) are higher in stage IV and also in cases having distant metastasis within the current work, there is no statistical correlation with clinicopathological parameters of studied GBC except with the age and the site of the tumor. HER2/neu positive were detected in 73.9% (17/23) of GBC cases aged over 50 years while only 11.8% (2/17) of cases under 50 years of age showed positive HER2/neu expression.
This is in agreement with Roa I et al., Doval DC et al., Pujani M et al., and Chow NH et al., [23-25,28]. It is also similar to Matsuyama S et al., who failed to find correlation with GBC grade [29].
Contradictory to present study results, correlation with advanced tumour stage and metastatic lymph node status was observed in studies done by Nakazawa K et al., and Puhalla H et al., [19,22], respectively. Kumari N et al., observed more HER2 /neu expression in well-differentiated GBC and advanced staged ones that disagrees with the current study [30].
The discrepancy between these findings and present study results may be explained by the use of different immunostaining procedures (e.g., monoclonal vs polyclonal antibodies and sensitivity of the technique), time of fixation, preservation of antigenic sites and number of samples analysed.
Mucins are high molecular weight glycoproteins that are produced by various epithelial cells. They are essential for epithelial cell protection and homeostasis, acting as an anti-adhesion molecule preventing tumour cell invasion [31]. The current work found there was significant difference of MUC1 expression concerning the pattern and intensity between the studied groups as low MUC1 expression within all cases of cholecytitis cases (25/25) and 90% (9/10) of metaplastic group, with apical surface polarisation while it was significantly increased in dysplastic lesion and GBC (high expression in 75% of both) with cytoplasmic localisation.
Concerning the relation with clinicopathological parameters, 90.9% (10/11) of poorly differentiated GBC cases showed high MUC1 expression compared with 22.2% (2/9) of well-differentiated ones (p<0.01), high MUC1 expression was in 84.6% (11/13) of pT3 and in only 20% (2/10) of pT1 with significant difference. The current study registered that 90.5% (19/21) of positive nodal metastatic cases and 87.5% (14/16) of distant metastatic GBC cases were of high MUC1 expression. Parallel to present study, Maurya KS et al., who found weak and apically located MUC1 expression in normal epithelial cells of control group in contrast to higher and extensive expression in malignant cells [32]. The loss of apical expression within malignant transformation leads to disturbance of cell-cell adhesion and facilitating the spread of carcinoma [32].
The results of Xiong L et al., Wang HH et al., Takagawa M et al., and Ghosh M et al., are similar to index findings concerning MUC1 overexpression in GBC [33-36]. Yamato T et al., study was in accordance with present study, as they reported rare expression of MUC1 in normal epithelial cells, while it was significantly higher in dysplastic and malignant tumour with depolarisation of its expression than in cholecystitis cases [37].
These findings are close to that of Bhoge A et al., [38] who registered positive expression rate of MUC1 was significantly higher in GBC (85%) and dysplastic lesion with cytoplasmic pattern (83%) while was not found in chronic cholecystitis [38]. In the same study, also there was significant correlation between MUC1 and advanced stage of GBC.
Based on that, present study emphasises some evidence about the pathogenesis of GBC which progresses from cholecystitis, metaplaia, dysplasia, and invasive cancer as seen in literature. MUC1 transcripts/protein are occurred with the sequel of GBC development from cholecystitis up to carcinoma passing by dysplasia, with significant variance in its intensity and distribution patterns [33,38].
Concerning Ki-67, 55% (22/40) of malignant group showed positive Ki-67 expression, with complete absence in submitted dysplastic and control group.
The current study recorded increasing Ki-67 expression with advancing stage of GBC with statistically significant correlation (p=0.012).
The current work found a significant correlation between the Ki-67 expression and the age of patients (p<0.01). Doval DC et al., and Lee CS, agree with present study concerning this observation [24,39] . Similarly, Singh KR et al., did not find significant correlation with grade and metastatic status of GBC cases [40]. On the other side, the same study registered positive Ki-67 expression in 55% of GBC cases and complete absence in cholecystitis group that also support present study results. Pujani M et al., found 60% malignant cases with Ki-67 positive staining that is in agreement with present study [25], but against us concerning the benign group that showed Ki-67 positive in 4% of benign gall bladder lesions.
No significant correlation was reported between the grade of differentiation and the wall infiltrate in relation to Ki-67 LI within the study done by Grau LH et al., similar to present study findings [15]. A noteworthy finding was that, Ki-67 expression (LI ≥20%) was observed in 56.3% (9/16) of cases with distant metastasis 57.1% (12/22) of positive lymph node metastatic cases compared, that justifies the more proliferation the more prevalent invasion and spread. Despite Lee CS approved the concerning significant correlation with the age and the stage of GBC patients but he found correlation with the grade that was not detected in the present study [39].
The continuous advance in targeted therapeutic approaches, that were directed against various cell adhesion molecules and cell cycle regulatory molecules, simultaneously with overexpression of HER2/neu and MUC1 in GBC put more light on the validation of both markers as a candidate in GBC targeted therapy, especially in advanced stage tumours as that of breast cancer. In the same time, applied markers were significantly different between tested groups as diagnostic markers declaring their role in GBC development [41].
Limitation(s)
The current study had potential limitations including the small size of dysplastic and malignant groups due to their rarity beside financial limitation.
Conclusion(s)
HER2/neu, and Ki-67 are overexpressed in GBS cases compared with control and dysplastic group with no significant correlation with the parameters except for age despite greater expression of both are more prevalent in advanced stage and cases with distant metastasis. Based on that, HER2/neu can be considered as a candidate for targeted therapy of GBC. Also, MUC1 core protein expression rate and pattern are significantly different within studied groups with statistical correlation with clinicopathological parameters of GBC suggesting that MUC1 core protein would be a marker of malignant transformation of gall bladder epithelium and its depolarised expression would also be a marker of GBC invasion. Subsequently, a new therapeutic agent can target these cell surface adhesion molecules (MUC1) in GBC treatment strategy.
**significant
WDC: Well differentiated carcinoma; MDC: Moderately Differentiated Carcinoma; PDC: Poorly differentiated carcinoma; LN: Lymph node; DM: Distant metastasis; **significant
WDC: Well differentiated carcinoma; PDC: Poorly differentiated carcinoma; LN: Lymph node; DM: Distant metastasis
WDC: Well differentiated carcinoma; MDC: Moderately differentiated carcinoma; PDC: Poorly differentiated carcinoma; LN: Lymph node; DM: Distant metastasis; **Significant