Introduction
Chronic Obstructive Pulmonary Disease (COPD) is characterised by airway obstruction leading to persistent respiratory symptoms, on account of exposure to noxious substances [1]. The primary aetiology of COPD is smoking, though in recent times, many other additional factors have also been identified. Patients with COPD complain of persistent cough, sputum production, and dyspnea [2]. A 90% of COPD related deaths have occurred in low-income countries [3]. In accordance with large epidemiological studies such as Burden of Obstructive Lung Disease (BOLD) showed that, with an increase in the prevalence of smoking in developing countries, the prevalence of COPD is expected to rise over the next 40 years [4,5]. There has been a somber forecast that, by 2060 over 5.4 million deaths would annually occur due to COPD and related conditions [6]. At present it is not curable, it can only ease symptoms, improve health-related quality of life, and reduce the risk of mortality [7]. Few studies have shown that “the intensity of systemic inflammation in severe COPD patients, including large numbers of neutrophils, macrophages, and lymphocytes is directly affects the health-related quality of life, airflow limitation, exercise intolerance, and comorbidities associated with COPD” [8-10].
The pharmacological treatment of COPD largely revolves around three categories of drugs namely inhaled corticosteroids, muscarinic antagonists, and β2-agonists [11]. According to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, the management of COPD depends on the severity of the disease and the rate of exacerbations leading to hospitalisation [12]. A fixed dose combination of fluticasone furoate, umeclidinium and vilanterol (Trelegy Ellipta/GlaxoSmithKline) is a newly developed triple combination medication approved for use by US FDA for severe COPD patients in September 2017. It has the distinction of being the first fixed-dose triple combination approved by US FDA [13]. This review summarises the evidence on the safety, efficacy and other important pharmacological properties of this unique triple combination FDC product for use in severe COPD.
Mechanism of Action
The triple combination inhaler contains fluticasone, umeclidinium and vilanterol. These drugs represent three different classes of the active component such as inhaled corticosteroid, antimuscarinic agent, and β2-agonist. The detailed mechanisms of action for the individual component are described below [Table/Fig-1,2].
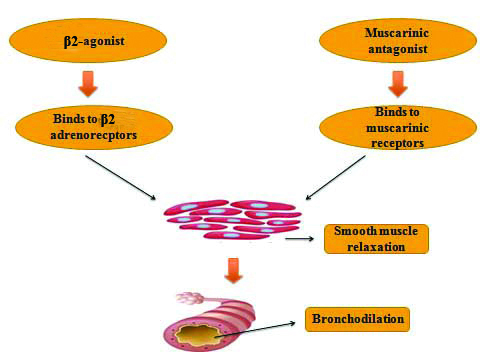
Represents the mechanism of β2-agonist and Muscarinic antagonist [14].
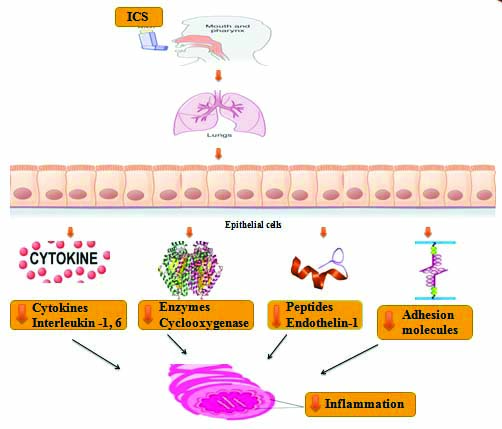
Represent the mechanism of Inhaled corticosteroids [15,16].
ICS: Inhaled corticosteroids
1) Fluticasone furoate
Fluticasone furoate is a potent glucocorticoid that is synthetic and chemically termed as triflourinated corticosteroid [17]. Corticosteroids play an essential role in the treatment of COPD, because of their excellent anti-inflammatory properties [18]. Fluticasone furoate binds with glucocorticoid receptors which help in the regulation of gene expression through glucocorticoid elements leading to inhibition of pro-inflammatory factors such as NF-KB [19,20]. It works by preserving epithelial integrity and permeability which occurs due to protease induced cell damage [Table/Fig-2].
2) Umeclidinium
Cholinergic parasympathetic nerves provide the pre-dominant innervations to the airway [21]. There are about 5 Muscarinic acetylcholine receptors, (mAChRs) M1 to M5 of which only M1 to M3 are well known in humans. These receptors are expressed abundantly in vagal nerve, pulmonary vasculature, smooth muscles, and sub mucosal glands in the lining of airway [22]. The mAChRs are single glycoprotein receptors combined by intra and extracellular loops which can be linked to ion channels (K+ or Ca2+). The binding of Acetylcholine ligand typically results in the activation of Adenylyl Cyclase (AC), activation of phospholipase C, and opening of the potassium channel. The released acetylcholine interacts with mAChRs to regulate contraction of smooth muscle in the airway, mucus secretion, and vasodilation. COPD is characterised by increased parasympathetic activity [23,24] which may be moderately reversed with anticholinergic agents such as umeclidinium and subsequently improve limitation in the airway [Table/Fig-1].
3) Vilanterol
Vilanterol is an effective bronchodilator that can ease the smooth muscle lining of airway [25]. They exert their effects by binding to the active site of β2-adrenoreceptors, which are present in abundance on smooth muscles [26]. The assumed cellular mode of action involves the signaling pathway such as activation of AC and production of intracellular cAMP, which in turn may activate the effector molecules of cAMP-dependent Protein Kinase A (PKA) and a Rap1, Epac guanine nucleotide exchange factor. PKA phosphorylates key regulatory proteins involved in the contraction of smooth muscle tone and cAMP which results in the sequestration of intracellular Ca2+, leading to relaxation of the smooth muscle [Table/Fig-1] [27].
Pharmacokinetics
1) Fluticasone furoate
When fluticasone furoate was administered by inhalation, maximum Serum concentration (Cmax) occurred within 0.5 to 1 hour. Bioavailability was 15.2%. Steady state was estimated to be achieved within six hours. The volume of distribution was 661L. It is highly bound to human plasma protein and principally metabolised through hepatic metabolism via CYP3A4. The elimination half-life of the drug was 24 hours and excreted through feces and urine [Table/Fig-3] [28].
Pharmacokinetics characteristics of fluticasone furoate, umeclidinium and vilanterol [28].
| Pharmacokinetic parameters | Fluticasone furoate | Umeclidinium | Vilanterol |
|---|
| Cmax | 0.5 to 1 hours | 5 to 15 minutes | 5 to 15 minutes |
| Bioavailability | 15.2% | 13% | 27.3% |
| Volume of distribution | 661L | 86L | 165L |
| Metabolism | Hepatic via CYP3A4 | Hepatic via CYP2D6 | Hepatic via CYP3A4 |
| Half life | 24 hours | 11 hours | 11 hours |
| Excretion | Principally in feces and urine | Feces (58%); Urine (22%) | Urine (70%); Feces (30%) |
Cmax: Maximum serum concentration
2) Umeclidinium
Upon inhalation of umeclidinium, Cmax was achieved within 5 to 15 minutes; Steady state was reached within 14 days. It binds to plasma protein and the volume of distribution was 86 L. It is metabolised through hepatic metabolism via CYP2D6. The elimination half-life of the drug was 11 hours and excreted through feces (58%) and urine (22%) [Table/Fig-3] [28].
3) Vilanterol
Cmax and steady state was achieved within 5 to 15 minutes, and 14 days respectively, upon inhalation of vilanterol. The drug is highly bound to plasma protein and has a distribution volume of 165L. It is metabolised by the liver via CYP3A4 with a half-life of 11 hours and excreted in urine (70%) and feces (30%) [Table/Fig-3] [28].
Efficacy
Overview of lung parameters and health related quality of life among COPD patients in major trials [Table/Fig-4] [29-33].
Overview of lung parameters and health related quality of life among COPD patients in major trial; + - Improved, X-Unchanged; SGRQ: St. George questionnaire; FEV1: Forced expiratory volume per second
| Study | Trough FEV1 | SGRQ | Rate of exacerbation | Rescue medication use |
|---|
| Siler TM et al., [29] (2015) | + | + | Not evaluated | + |
| Lipson DA et al., [30] (2018) | + | + | + | Not evaluated |
| Lipson DA et al., [31] (2017) | + | + | + | Not evaluated |
| Bremner PR et al., [32] (2018) | + | X | X | Not evaluated |
| Tabberer M et al., [33] (2018) | Not evaluated | + | Not evaluated | + |
The Lung Function and Quality of Life Assessment in COPD with Closed Triple Therapy (FULFIL) trial evaluated the Trelegy Ellipta (GSK) (fluticasone furoate/umeclidinium/vilanterol) against budesonide/formoterol combination in patients with COPD, GOLD class D having an FEV1 <50% and COPD Assessment test ≥10 and having a moderate or severe exacerbation in the past one year. Tough FEV1 and St. George’s Respiratory questionnaire total score showed remarkable improvement at the end of 24 weeks of therapy in the triple therapy group compared to the control group. Triple therapy had greater odds of favorable response in comparison to control with respect to SGRQ improvement. The annualised rate of exacerbations was lesser in triple therapy [31].
In 2018, results of InforMing the PAthway of COPD Treatment (IMPACT) trial were published, which compared Trelegy ellipta (GSK) once-daily single inhaler triple combination (fluticasone furoate/umeclidinium/vilanterol) versus dual therapies i.e., fluticasone furoate/vilanterol or umeclidinium/vilanterol. It was a phase III, randomised trial with a total of 10,355 patients enrolled as intent to treat. The primary endpoint of the study was met, in terms of lower exacerbation rate in the triple combination therapy, when compared to either of the dual therapies. However, it is possible that the abrupt withdrawal of ICS in patients of the LABA/LAMA group could have triggered the increased risk of exacerbations when compared to the other groups. The annual hospital admissions due to severe exacerbations were significantly reduced. There was a high risk of pneumonia in triple therapy compared to umeclidinium/vilanterol. A trend towards lesser all-cause mortality in the triple therapy was reported, however finding needs to be explored further as it did not achieve statistical significance, due to the exploratory nature of the endpoint. It is speculated that the immunosuppressive effects of ICS increase the risk of pneumonia but its anti-inflammatory effect can mitigate the severity of pneumonia, thereby reducing the mortality [30].
Safety
The adverse effects of triple combination therapy are a reflection of the individual components of the inhaler. For instance, vilanterol by virtue of its sympathetic activation can cause tachycardia, a rise in blood pressure, and cardiac arrhythmia including supraventricular tachycardia and extra systoles [34]. One should be extremely cautious when prescribing this combination in patients with hypertension or coronary artery disease. Patients with thyrotoxicosis or seizure disorder are more prone to the worsening of the disease with vilanterol [35]. ICS is known to potentially cause worsening of intraocular pressure, glaucoma, and increase the risk of cataracts among COPD patients. Some of these issues such as glaucoma worsening can occur even with umeclidinium due to its anticholinergic property. An increased predilection to Candida infection of the oral cavity can be reduced with proper rinsing of the mouth to a certain extent [36]. Paradoxical bronchospasm, reduction in bone mineral density, adrenal suppression are some of the other troublesome effects that can be potentially seen among users of the triple combination. Headache, diarrhea, cough, dyspepsia, and back pain are some of the minor adverse events associated with the triple combination inhaler [28].
Current Status and Future Perspectives
Currently, the triple combination of umeclidinium/vilanterol/fluticasone is approved in patients with COPD who are already consuming LABA-LAMA combination or LABA-ICS combination and still not achieving adequate control [37]. The drug is also indicated in patients who have been using fluticasone-vilanterol combination and umeclidinium as two separate medications, as using the triple therapy would enhance compliance due to convenience and also helps the patients to stick to the treatment regimen to avoid exacerbation, and persistent symptoms [38]. The triple combination is available as a dry powder inhaler. Besides this, another triple therapy comprising of budesonide, glycopyrronium, and formoterol has also been approved by the European Medicines agency (EMA) based on the positive results from TRIBUTE study. In this study, a single inhaler triple combination of Beclomethasone Dipropionate, Formoterol Fumarate, and Glycopyrronium (BDP/FF/GLY) compared with single inhaler dual combination of indacaterol and glycopyrronium (IND/GLY). It showed BDP/FF/GLY reduced exacerbations rate compared to IND/GLY [Table/Fig-5] [39,40].
Characteristics of Trelegy Ellipta and Trimbow [28,40].
| Name of the drugs | Fluticasone/umeclidinium/vilanterol | Beclometasone/formoterol/glycopyrronium |
|---|
| Mode of delivery | Dry powder inhaler | Metered-dose inhaler |
| Dose | FF 100 mcg/umec 62.5 mcg/vi 25 mcg | BDP 87 mcg/FF 5 mcg/GP 9 mcg |
| Frequency | Once daily | Twice daily |
| Brand name | Trelegy Ellipta | Trimbow |
| Manufacturer | GSK/Innoviva Inc. | ChiesiFarmaceutici |
| Cost for 28 days | £41.53 | £41.53 |
| Approved by | FDA (Sep 2017), EMA (Nov 2017), NMPA (Nov 2019) | EMA (July 2017) |
FDA: Food and drug administration; EMA: European medicines agency; NMPA: National medical products administration
It remains to be seen how these two inhalers would fare against each other in head to head trials in future. There has not been a piece of definite evidence to prove that providing the same drugs in a single inhaler is superior to giving them the drugs separately. Nevertheless, there are data to suggest that within one year of diagnosis; almost 32% of patients require triple therapy eventually [41]. It has also been suggested that in a new COPD patient who has been hospitalised for acute exacerbations, there is a strong case for providing them on triple therapy after discharge from the hospital for a few months at least. The drugs could be gradually tapered subsequently for these patients based on the response of the patient [42]. However, this has to be evaluated in a trial setting. All scenarios that warrant avoiding ICS in COPD patients apply even for triple therapy. For instance, HIV patients receiving Highly Active Antiretroviral Therapy (HAART) may have the metabolism of ICS impaired due to hepatic enzyme inhibition potential, resulting in an increased predilection for Cushing’s syndrome. Patients with active tuberculosis or any other infection should also not be prescribed ICS, and hence triple therapy [43]. Despite these challenges, triple therapy is bound to be increasingly used in advanced COPD owing to its convenience from the patient’s vantage point [44]. Finally, in terms of cost analysis, Fluticasone Furoate/Umeclidinium/Vilanterol (FF/UMEC/VI) established a cost-effective treatment option for patients with COPD when compared to treatment with Beclometasone/formoterol/glycopyrronium [45-47].
Conclusion(s)
The pharmacological management of patients with COPD continues to remain daunting in patients with advanced stage of disease. Besides LAMA, LABA, and ICS there have not been any other drugs that have revolutionised the care of these patients. The availability of a single inhaler device for delivering a triple combination of LABA/LAMA/ICS is a small success story in the quest to identify better therapies for patients with COPD, who are so prone to repeat acute exacerbations which could eventually turn fatal. With appropriate use of the medication, the combination of umeclidinium/vilanterol/fluticasone could add to the limited arsenal in the fight against COPD.
Cmax: Maximum serum concentration
FDA: Food and drug administration; EMA: European medicines agency; NMPA: National medical products administration
[1]. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease [Internet]. Goldcopd.org. 2020 [cited 9 May 2020]. Available from: www.goldcopd.org/wp-content/uploads/2020/03/GOLD-2020-POCKET-GUIDE-ver1.0_FINAL-WMV.pdf [Google Scholar]
[2]. Wacker ME, Kitzing K, Jörres RA, Leidl R, Schulz H, Karrasch S, The contribution of symptoms and comorbidities to the economic impact of COPD: An analysis of the German COSYCONET cohortInt J Chron Obstruct Pulmon Dis 2017 12:3437-48.10.2147/COPD.S14185229270005 [Google Scholar] [CrossRef] [PubMed]
[3]. Chronic obstructive pulmonary disease (COPD) [Internet]. Who.int. 2020 [cited 9 May 2020]. Available from: www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) [Google Scholar]
[4]. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence studyLancet 2007 370:741-50.10.1016/S0140-6736(07)61377-4 [Google Scholar] [CrossRef]
[5]. GBD 2016 Risk Factors CollaboratorsGlobal, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016Lancet 2017 390(10100):1345-422.10.1016/S0140-6736(17)32366-8 [Google Scholar] [CrossRef]
[6]. Salvi S, Kumar GA, Dhaliwal RS, Paulson K, Agrawal A, Koul PA, The burden of chronic respiratory diseases and their heterogeneity across the states of India: The Global Burden of Disease Study 1990-2016The Lancet Global Health 2018 6(12):e1363-74. [Google Scholar]
[7]. Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P, Depressive symptoms and chronic obstructive pulmonary disease: Effect on mortality, hospital readmission, symptom burden, functional status, and quality of lifeArchives of Internal Medicine 2007 167(1):60-67.10.1001/archinte.167.1.6017210879 [Google Scholar] [CrossRef] [PubMed]
[8]. Barnes PJ, Celli BR, Systemic manifestations and comorbidities of COPDEur Respir Rev 2009 33(5):1165-85.10.1183/09031936.0012800819407051 [Google Scholar] [CrossRef] [PubMed]
[9]. Agustí A, Systemic effects of chronic obstructive pulmonary disease: What we know and what we don’t know (but should)Proc Am Thorac Soc 2007 4:522-25.10.1513/pats.200701-004FM17878464 [Google Scholar] [CrossRef] [PubMed]
[10]. Garcia-Rio F, Miravitlles M, Soriano JB, Muñoz L, Duran-Tauleria E, Sánchez G, Systemic inflammation in chronic obstructive pulmonary disease: A population-based studyRespir Res 2010 11(63):01-15.10.1186/1465-9921-11-6320500811 [Google Scholar] [CrossRef] [PubMed]
[11]. Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, Long-Acting Muscarinic Antagonist (LAMA) plus Long-Acting Beta-Agonist (LABA) versus LABA plus Inhaled Corticosteroid (ICS) for stable Chronic Obstructive Pulmonary Disease (COPD)Cochrane Database Syst Rev 2017 2(2):CD01206610.1002/14651858.CD012066.pub228185242 [Google Scholar] [CrossRef] [PubMed]
[12]. Russi EW, Karrer W, Brutsche M, Eich C, Fitting JW, Frey M, Diagnosis and management of chronic obstructive pulmonary disease: The Swiss guidelinesRespiration 2013 85(2):160-74.10.1159/00034602523406723 [Google Scholar] [CrossRef] [PubMed]
[13]. Albertson TE, Murin S, Sutter ME, Chenoweth JA, The Salford Lung Study: A pioneering comparative effectiveness approach to COPD and asthma in clinical trialsPragmatic and Observational Research 2017 8:175-81.10.2147/POR.S14415729033625 [Google Scholar] [CrossRef] [PubMed]
[14]. DeBellis RJ, Mechanism of action of long-acting bronchodilatorsClin Pulm Med 2005 12(4):S10-12.10.1097/01.cpm.0000170112.09340.1a [Google Scholar] [CrossRef]
[15]. Williams DM, Clinical pharmacology of corticosteroidsRespiratory Care 2018 63(6):655-70.10.4187/respcare.0631429794202 [Google Scholar] [CrossRef] [PubMed]
[16]. Barnes PJ, Inhaled CorticosteroidsPharmaceuticals (Basel) 2010 3(3):514-40.10.3390/ph303051427713266 [Google Scholar] [CrossRef] [PubMed]
[17]. FDA. VeramystTM (fluticasone furoate) nasal spray: |US prescribing information [online] Accessed 2020 Nov 05 [Google Scholar]
[18]. Suissa S, McGhan R, Niewoehner D, Make B, Inhaled corticosteroids in chronic obstructive pulmonary diseaseAnn Am Thorac Soc 2007 4(7):535-42.10.1513/pats.200701-024FM17878466 [Google Scholar] [CrossRef] [PubMed]
[19]. Horvath G, Wanner A, Inhaled corticosteroids: Effects on the airway vasculature in bronchial asthmaEur Respir Rev 2006 27(1):172-87.10.1183/09031936.06.0004860516387951 [Google Scholar] [CrossRef] [PubMed]
[20]. Ericson-Neilsen W, Kaye AD, Steroids: Pharmacology, complications, and practice delivery issuesOchsner Journal 2014 14(2):203-07. [Google Scholar]
[21]. Belmonte KE, Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary diseaseAnn Am Thorac Soc 2005 2(4):297-304.10.1513/pats.200504-043SR16267352 [Google Scholar] [CrossRef] [PubMed]
[22]. Babu KS, Morjaria JB, Umeclidinium in chronic obstructive pulmonary disease: Latest evidence and place in therapyTherapeutic Advances in Chronic Disease 2017 8(4-5):81-91.10.1177/204062231770082228491268 [Google Scholar] [CrossRef] [PubMed]
[23]. Wessler IK, Kirkpatrick CJ, The nonneuronal cholinergic system: An emerging drug target in the airwaysPulm Pharmacol Ther 2001 14:423-34.10.1006/pupt.2001.031311782122 [Google Scholar] [CrossRef] [PubMed]
[24]. Gosens R, Zaagsma J, GrootteBromhaar M, Nelemans A, Meurs H, Acetylcholine: A novel regulator of airway smooth muscle remodelling?Eur J Pharmacol 2004 500:193-201.10.1016/j.ejphar.2004.07.02515464033 [Google Scholar] [CrossRef] [PubMed]
[25]. Cazzola M, Page CP, Rogliani P, Matera MG, β2-agonist therapy in lung diseaseAm J Respir Crit Care Med 2013 187(7):690-96.10.1164/rccm.201209-1739PP23348973 [Google Scholar] [CrossRef] [PubMed]
[26]. Billington CK, Ojo OO, Penn RB, Ito S, cAMP regulation of airway smooth muscle functionPulm Pharmacol Ther 2013 26:112-20.10.1016/j.pupt.2012.05.00722634112 [Google Scholar] [CrossRef] [PubMed]
[27]. Billington CK, Hall IP, Novel cAMP signalling paradigms: Therapeutic implications for airway diseaseBr J Pharmacol 2012 166:401-10.10.1111/j.1476-5381.2011.01719.x22013890 [Google Scholar] [CrossRef] [PubMed]
[28]. US Food and Drug Administration. Trelegy®Ellipta® highlights of prescribing information. Updated September 2017 [cited 9 May 2020]. https://www.accessdata.fda.gov/drugs at fda _docs/label /2017/20948 2s000 lbl.pdf [Google Scholar]
[29]. Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A, Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: Results of two randomised studiesRespir Med Res 2015 109(9):1155-63.10.1016/j.rmed.2015.06.00626117292 [Google Scholar] [CrossRef] [PubMed]
[30]. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Once-daily single-inhaler triple versus dual therapy in patients with COPDN Engl J Med 2018 378(18):1671-80.10.1056/NEJMoa171390129668352 [Google Scholar] [CrossRef] [PubMed]
[31]. Lipson DA, Barnacle H, Birk R, Brealey N, Locantore N, Lomas DA, FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med 2017 196(4):438-46.10.1164/rccm.201703-0449OC28375647 [Google Scholar] [CrossRef] [PubMed]
[32]. Bremner PR, Birk R, Brealey N, Ismaila AS, Zhu CQ, Lipson DA, Single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: A randomised non-inferiority studyRespir Med Res 2018 19(1):1910.1186/s12931-018-0724-029370819 [Google Scholar] [CrossRef] [PubMed]
[33]. Tabberer M, Lomas DA, Birk R, Brealey N, Zhu CQ, Pascoe S, Once-daily triple therapy in patients with COPD: patient-reported symptoms and quality of lifeAdvances in Therapy 2018 35(1):56-71.10.1007/s12325-017-0650-429313286 [Google Scholar] [CrossRef] [PubMed]
[34]. Vestbo J, Leather D, Barkerly ND, New J, Gibson JM, McCorkindale S, Effectiveness of Fluticasone Furoate-Vilanterol for COPD in Clinical PracticeN Engl J Med 2016 375(13):1253-60.10.1056/NEJMoa160803327593504 [Google Scholar] [CrossRef] [PubMed]
[35]. Lotvall J, Bateman ED, Bleecker ER, Busse WW, Woodcock A, Follows R, 24-h duration of the novel LABA vilanterol trifenatate in asthma patients treated with inhaled corticosteroidsERJ Open Res 2012 40:570-79.10.1183/09031936.0012141122362859 [Google Scholar] [CrossRef] [PubMed]
[36]. Tashkin DP, Strange C, Inhaled corticosteroids for chronic obstructive pulmonary disease: What is their role in therapy?Int J Chron Obstruct Pulmon Dis 2018 13:2587-601.10.2147/COPD.S17224030214177 [Google Scholar] [CrossRef] [PubMed]
[37]. Malerba M, Nardin M, Santini G, Mores N, Radaeli A, Montuschi P, Single-inhaler triple therapy utilizing the once-daily combination of fluticasone furoate, umeclidinium and vilanterol in the management of COPD: The current evidence base and future prospectsTher Adv Respir Dis 2018 12:175346661876077910.1177/175346661876077929537340 [Google Scholar] [CrossRef] [PubMed]
[38]. Gaduzo S, McGovern V, Roberts J, Scullion JE, Singh D, When to use single-inhaler triple therapy in COPD: A practical approach for primary care health care professionalsInt J Chron Obstruct Pulmon Dis 2019 14:391-401.10.2147/COPD.S17390130863039 [Google Scholar] [CrossRef] [PubMed]
[39]. Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trialLancet 2018 391(10125):1076-84.10.1016/S0140-6736(18)30206-X [Google Scholar] [CrossRef]
[40]. Trimbow [Internet]. Ema.europa.eu. 2020 [cited 9 November 2020]. Available from: www.ema.europa.eu/en/documents/product-information/trimbow-epar-product-information_en.pdf [Google Scholar]
[41]. Brusselle G, Price D, Gruffydd-Jones K, Miravitlles M, Keininger DL, Stewart R, The inevitable drift to triple therapy in COPD: An analysis of prescribing pathways in the UKInt J Chron Obstruct Pulmon Dis 2015 10:2207-17.10.2147/COPD.S9169426527869 [Google Scholar] [CrossRef] [PubMed]
[42]. Reis AJ, Alves C, Furtado S, Ferreira J, Drummond M, Robalo-Cordeiro C, COPD exacerbations: Management and hospital dischargePulmonology 2018 24(6):345-50.10.1016/j.pulmoe.2018.06.00630049647 [Google Scholar] [CrossRef] [PubMed]
[43]. Raveendran AV, Inhalational Steroids and Iatrogenic Cushing’s SyndromeThe Open Respir Med Res 2014 8(1):74-84.10.2174/187430640140801007425674177 [Google Scholar] [CrossRef] [PubMed]
[44]. Vanfleteren LE, Ullman A, Nordenson A, Andersson A, Andelid K, Fabbri LM, Triple therapy (ICS/LABA/LAMA) in COPD: Thinking out of the boxERJ Open Research 2019 5(1):00185-2018.10.1183/23120541.00185-201830775372 [Google Scholar] [CrossRef] [PubMed]
[45]. Ismaila AS, Risebrough N, Schroeder M, Shah D, Martin A, Goodall EC, Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: The IMPACT trialInt J Chron Obstruct Pulmon Dis 2019 14:268110.2147/COPD.S21607231819401 [Google Scholar] [CrossRef] [PubMed]
[46]. Schroeder M, Benjamin N, Atienza L, Biswas C, Martin A, Whalen JD, Cost-effectiveness analysis of a once-daily single-inhaler triple therapy for patients with Chronic Obstructive Pulmonary Disease (COPD) using the FULFIL trial: A Spanish perspectiveInt J Chron Obstruct Pulmon Dis 2020 15:162110.2147/COPD.S24055632764908 [Google Scholar] [CrossRef] [PubMed]
[47]. Schroeder M, Shah D, Risebrough N, Martin A, Zhang S, Ndirangu K, Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: A UK perspectiveRespir Med X 2019 1:10000810.1016/j.yrmex.2019.100008 [Google Scholar] [CrossRef]