Tentorium cerebelli is a crescent shaped fold of dura mater that roofs over the posterior cranial fossa dividing the cranial cavity into a supratentorial compartment, containing the forebrain and infratentorial compartment, containing the hindbrain. Tentorium cerebelli is deficient centrally and anteriorly producing a gap, the tentorial notch [1]. Midbrain passes through the tentorial notch and this notch provides the only communication between the supratentorial and infratentorial compartments. The area between the brainstem and free tentorial edge is divided into the anterior, middle, and posterior incisural spaces. The anterior incisural space is located anterior to the brainstem and oculomotor nerves traverse through it; the middle incisural space is located on each side of the brainstem and is closely related to the hippocampus; and the posterior incisural space is situated posterior to the midbrain and corresponds to the region of the pineal gland and vein of Galen [2].
The tentorial aperture is a complex space that varies notably in size and shape and it has significant clinical importance in the field of neurosurgery. Although this space is demarcated by the free edge of the tentorium cerebelli, it has remained anatomically elusive because of its three-dimensional anatomy, absence of blood vessels running along its edge, and only occasional calcification [3]. Before the era of neuroimaging, clinicians used to rely on clinicopathological diagnosis for neurological conditions. The lethal consequences of such brainstem compression due to transtentorial coning were first recognised and described by Meyer A [4]. Quantification of this tentorial aperture remained a challenge for neurosurgeons until milestone studies performed by Corsellis JA and Sunderland S shed some light on anatomical variations in the tentorial notch [5,6]. There remained a lacuna in the percipience of diverse clinical presentations of herniation syndromes in patients with otherwise indistinguishable intracranial conditions. This void has been filled upto some extent with emergence of modern neuroimaging modalities [7], but literature, so far available, is remarkably devoid of substantial observations on the morphometry of the tentorial notch.
In-depth understanding of anatomical variations in tentorial hiatus may help to explain the progression and clinical outcomes of transtentorial herniations and brainstem distortion in concussive and inertial injuries. So, the present anatomical study was conducted with the aim of elucidating the morphometry of the tentorial notch in Indian population in autopsy specimens. Emphasis was laid on precise dissection to measure tentorial notch dimensions and relations of various neurological structures within the tentorial notch. This study provides a baseline anatomical data for determination of notch type on Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) preoperatively which may influence the criteria for interventional procedures in neurosurgery.
Materials and Methods
This descriptive cross-sectional study was performed from August, 2010 to January, 2012 after getting an approval from Institutional Review Board. It was conducted in the Department of Anatomy in collaboration with Forensic Medicine. The study was carried out during medico-legal autopsies of 40 unfixed adult human cadavers, age ranging from 20 to 65 years. Cases, where autopsies performed within 24 hours of death, were included to avoid any alteration in the tentorial notch because of decomposition or putrefaction [8]. Cases with any primary neurological cause of death such as traumatic head injury, cerebrovascular accident, infection and space occupying lesion, cases with any intracranial surgical intervention and cases in which structures to be seen were distorted before or during autopsy were excluded from the study.
Steps of Dissection
Unfixed head of the cadaver was placed on a wooden block at an angle of 45° to 60° above the horizontal plane. An incision across the vertex was made from mastoid to mastoid in the coronal plane and the scalp was reflected anteriorly up to orbit and posteriorly below the occipital protuberance. Periosteum was denuded and temporal muscles were dissected off. Skull cap was removed by making a cut with an electric saw that extended circumferentially little above the eyebrow ridges and little above the inion. The dura mater was cut at the level of removal of skull cap. The frontal lobes were lifted gently with the fingers of the left hand and falx cerebri was cut from its attachment on crista galli and reflected posteriorly keeping it intact. The diencephalon was cut axially above the level of optic chiasma, through the third ventricle to the apex of tentorial notch leaving intact a portion of diencephalon, posterior portion of falx and the tentorium. The cerebral hemispheres were removed, without distorting the anatomy of tentorium. Optic nerves were cut cranial to the sella turcica. The optic chiasma was lifted and mesencephalon was cut in the axial plane following the contour of the tentorial edge to the point of notch apex. Field was irrigated with water to remove fresh blood [9].
Measurement of Parameters
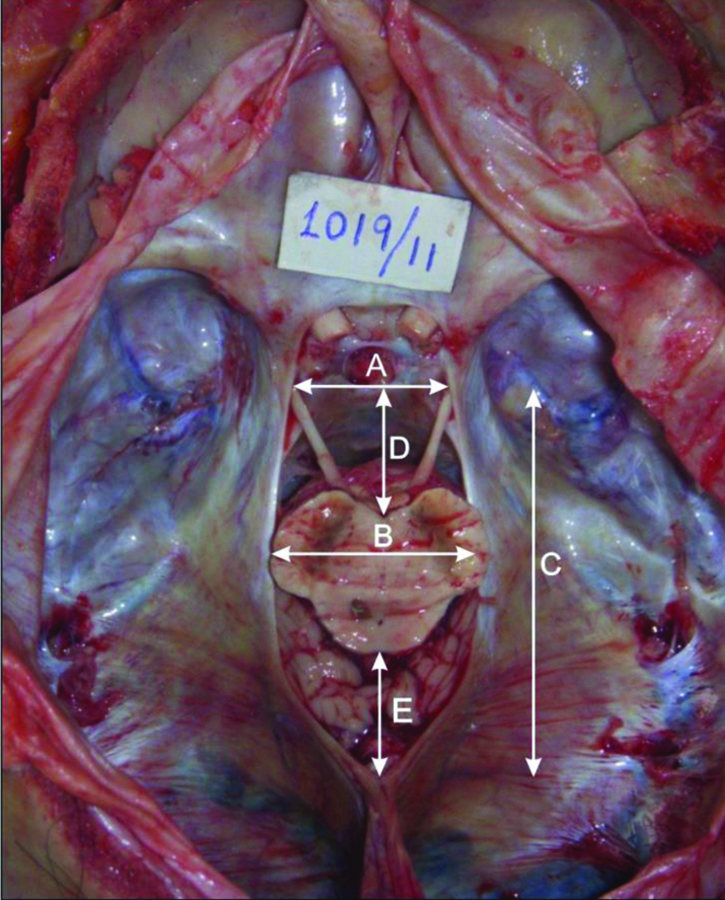
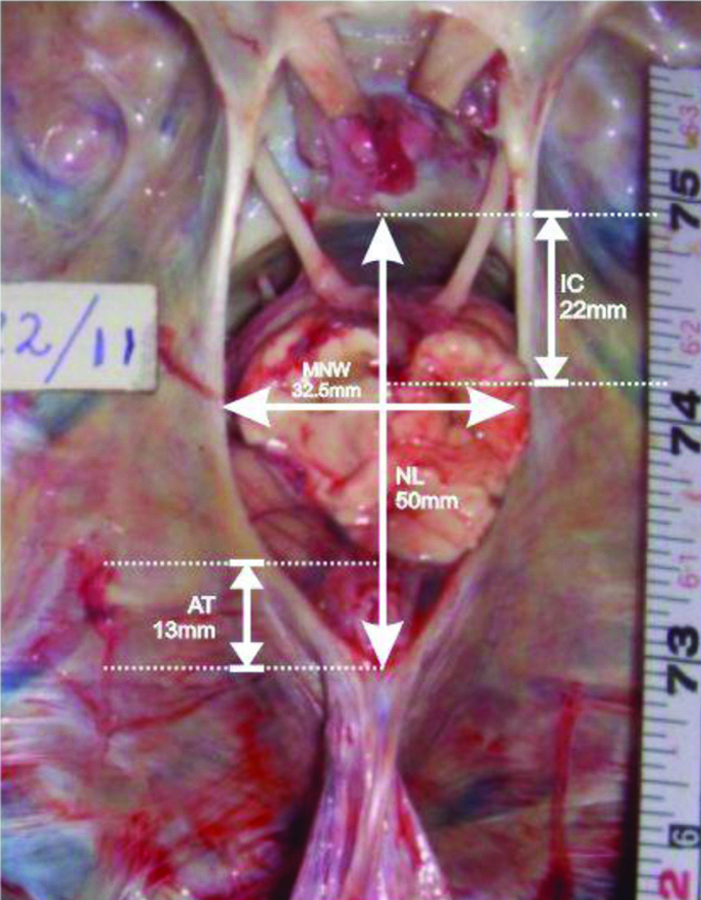
All the measurements were taken insitu with the help of Vernier Calipers. The following parameters were measured [Table/Fig-1]: 1) ANW, the width of the tentorial notch through the posterior aspect of the dorsum sellae; 2) MNW, the maximum width of the tentorial notch in axial plane; 3) NL, the distance between posterosuperior edge of the dorsum sellae in the mid-plane and the apex of the notch; 4) IC distance, the distance from the interpeduncular fossa to the posterosuperior edge of the dorsum sellae; 5) AT distance, the distance between the tectum of midbrain in the mid- plane and the apex of tentorial notch.
Photograph showing measurements of tentorial notch obtained in this study.
ANW: Anterior notch width (A); MNW: Maximum notch width (B); NL: Notch length (C); IC: Interpedunculoclival distance (D); AT: Apicotectal distance (E)
Statistical Analysis
The data obtained was subjected to statistical analysis using SPSS version 21.0 (Armonk, NY: IBM Corp.). The tentorial notches were categorised into eight types by following Adler DE and Thomas HM [9]. By applying quartile distribution technique to MNW, tentorial notches were divided into narrow, midrange and wide type. By applying quartile distribution technique to NL, tentorial notches were divided into short, midrange and long type. Applying quartile cut off points to IC distance, the positions of brainstem within the tentorial notch were labeled as prefixed, midposition and postfixed [9]. Pearson’s correlation test and significant two-tailed test were applied to ascertain the correlation between the various data sets. The p-value <0.05 were considered as statistically significant.
Results
ANW, MNW, NL, IC and AT Distance
Descriptive analysis of all the parameters obtained in this study is summarised in [Table/Fig-2]. The mean values of the ANW and MNW were measured 26.92±2.14 mm (range 22-31.5) and 29.77±2.26 mm (24-34 mm), respectively, and a strong positive correlation between these two values was observed (r=0.72, p<0.001). No statistically significant correlation was observed between other variables [Table/Fig-3]. First quartile of MNW ranging from 24.0 to 28.8 mm labeled as narrow, middle two quartiles ranging from 28.9 to 31.1 mm labeled as midrange and last quartile ranging from 31.2 to 34.0 mm was labeled as wide type. Out of 40 cases, 10 were narrow (25%), 20 were midrange (50%) and 10 were wide (25%). First quartile of NL between 50.0-55.3 mm was labeled as short (25%, n=10), middle two quartiles between 55.4-59.6 mm were labeled as midrange (50%, n=20) and last quartile of NL between 59.7-68.0 mm was labeled as long (25%, n=10).
Descriptive analysis of morphometric parameters of tentorial notch (n=40).
| Value | ANW (mm) | MNW (mm) | NL (mm) | IC distance (mm) | AT distance (mm) |
|---|
| Valid | 40 | 40 | 40 | 40 | 40 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Mean | 26.92 | 29.77 | 57.98 | 21.21 | 25.81 |
| Standard error of mean | 0.34 | 0.36 | 0.71 | 0.59 | 1.27 |
| Median | 27.00 | 29.50 | 58.00 | 22.00 | 24.00 |
| Mode | 26.00 | 29.00 | 59.00 | 17.00 | 23.00 |
| Standard deviation | 2.14 | 2.26 | 4.52 | 3.72 | 8.04 |
| Variance | 4.61 | 5.13 | 20.41 | 13.84 | 64.74 |
| Range | 9.50 | 10.00 | 18.00 | 16.50 | 29.00 |
| Minimum | 22.00 | 24.00 | 50.00 | 10.00 | 13.00 |
| Maximum | 31.50 | 34.00 | 68.00 | 26.50 | 42.00 |
| 1st quartile | 26.00 | 28.80 | 55.30 | 17.50 | 18.80 |
| 3rd quartile | 28.00 | 31.10 | 59.60 | 24.10 | 31.20 |
ANW: Anterior notch width, MNW: Maximum notch width, NL: Notch length, IC: Interpedunculoclival, AT: Apico-tectal
Pearson correlation and significant two-tailed test.
| | ANW | MNW | NL | IC distance | AT distance |
|---|
| ANW | PC | 1 | 0.720* | -0.110 | 0.085 | -0.039 |
| Sig. | - | <0.001 | 0.500 | 0.600 | 0.809 |
| MNW | PC | 0.720* | 1 | -0.143 | 0.122 | -0.149 |
| Sig. | <0.001 | - | 0.378 | 0.452 | 0.360 |
| NL | PC | -0.110 | -0.143 | 1 | 0.150 | 0.136 |
| Sig. | 0.500 | 0.378 | - | 0.354 | 0.402 |
| IC Distance | PC | 0.085 | 0.122 | 0.150 | 1 | 0.190 |
| Sig. | 0.600 | 0.452 | 0.354 | - | 0.239 |
| AT Distance | PC | -0.039 | -0.149 | 0.136 | 0.190 | 1 |
| Sig. | 0.809 | 0.360 | 0.402 | 0.239 | - |
| N | 40 | 40 | 40 | 40 | 40 |
PC: Pearson correlation; Sig: Significant two-tailed test; *Correlation is significant at the 0.001 level (2-tailed); ANW: Anterior notch width; MNW: Maximum notch width; NL: Notch length, AT: Apicotectal, IC: Interpedunculoclival
Brainstem position was labeled as prefixed brainstem (27.5%, n=11) when the IC distance was between 10.0-17.5 mm, as midposition brainstem (47.5%, n=19) when IC distance was between 17.6-24.1 mm and as postfixed brainstem (25%, n=10) when IC distance was between 24.2-26.5 mm. By applying quartile analysis to AT distance, it was observed that the first quartile labeled as short type AT distance varied from 13.0 mm to 18.8 mm including 25% cases (n=10). The second and third quartiles together termed as midrange type AT distance, varied from 18.9 mm to 31.2 mm, including 50% cases (n=20). The last quartile, termed a long type AT distance, varied from 31.3 mm to 42.0 mm which included 25% (n=10) of the total cases. The amount of cerebellar parenchyma exposed in posterior incisural space was not quantified, but a positive visual correlation was observed between exposed cerebellar tissue and the AT distance.
Classification of Tentorial Notch
Using the variables, NL and MNW, tentorial notches were categorised into eight types. [Table/Fig-4] shows the matrix formation and classification of the tentorial notch into eight types. [Table/Fig-5] summarises the range of the measurements used to categorise the tentorial notch and percentage of the different types of notches. Tentorial notches with wide MNW (≥31.2 mm) and a midrange NL were characterised as wide [Table/Fig-6], and tentorial notches with narrow MNW (≤28.8 mm) and a midrange NL were characterised as Narrow. Tentorial notches with long NL (≥59.7 mm) and a midrange MNW were characterised as Long (7.5% of cases), and tentorial notches with short NL (≤55.3 mm) and a midrange MNW were characterised as short (12.5% of cases). Tentorial notches with a midrange MNW and midrange NL were labeled as Typical type [Table/Fig-7]. Tentorial notches with wide MNW and long NL were labeled as Large (5% of cases), and tentorial notches with narrow MNW and short NL were labeled as small (5% of cases). Some tentorial notches were found to be both wide and short (7.5%) or long and narrow (12.5%). Both types of notches were characterised as Mixed [Table/Fig-5,8,9].
Matrix formation used for tentorial notch classification
| Type of notch (No. of cadavers), n= 40 |
|---|
| MNW | Short NL | Midrange NL | Long NL |
| Narrow | Small (2) | Narrow (3) | Mixed (5) |
| Midrange | Short (5) | Typical (12) | Long (3) |
| Wide | Mixed (3) | Wide (5) | Large (2) |
MNW: Maximum notch width; NL: Notch length
Types of tentorial notch and criteria used for typing.
| Type of notch | Dimension | Range in mm | Percentage |
|---|
| Wide | MNW (wide) | 31.2-34.0 | 12.5 |
| NL (midrange) | 55.4-59.6 |
| Narrow | MNW (narrow) | 24.0-28.8 | 7.5 |
| NL (midrange) | 55.4-59.6 |
| Long | MNW (midrange) | 28.9-31.1 | 7.5 |
| NL (long) | 59.7-68.0 |
| Short | MNW (midrange) | 28.9-31.1 | 12.5 |
| NL (short) | 50.0-55.3 |
| Typical | MNW (midrange) | 28.9-31.1 | 30 |
| NL (midrange) | 55.4-59.6 |
| Large | MNW (wide) | 31.2-34.0 | 5 |
| NL (long) | 59.7-68.0 |
| Small | MNW (narrow) | 24.0-28.8 | 5 |
| NL (short) | 50.0-55.3 |
| Mixed | MNW (narrow) | 24.0-28.8 | 12.5 |
| NL (long) | 59.7-68.0 |
| MNW (wide) | 31.2-34.0 | 7.5 |
| NL (short) | 50.0-55.3 |
MNW: Maximum notch width, NL: Notch length
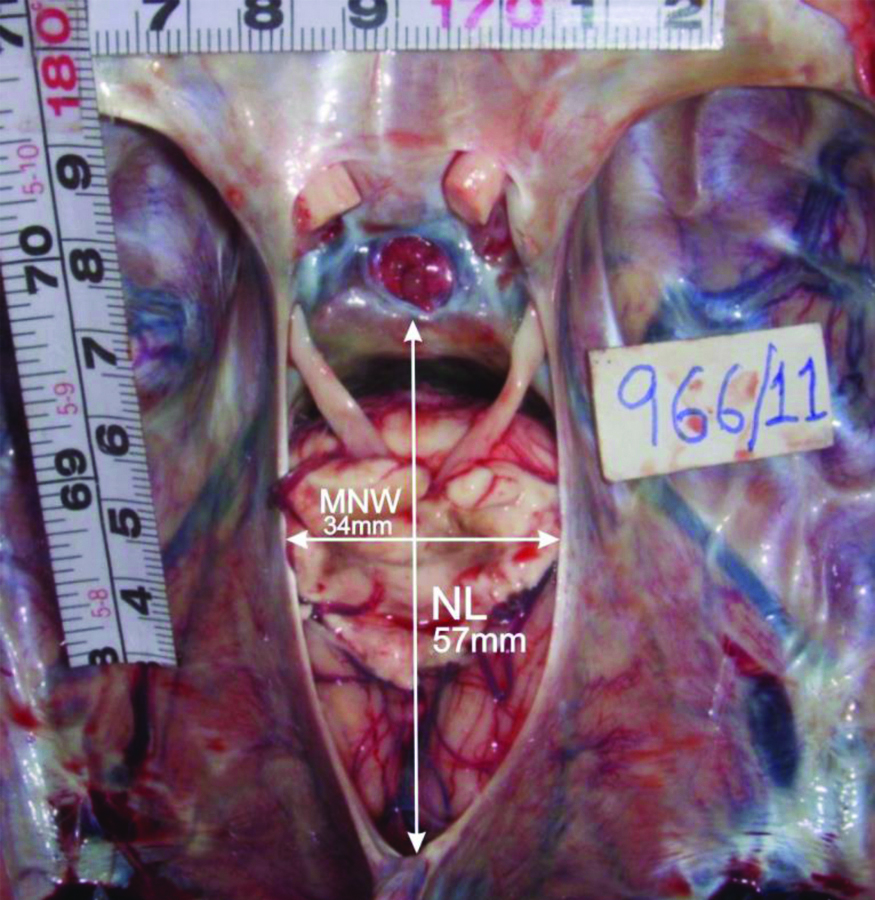
Photograph showing wide type of notch (wide MNW and midrange NL) and mid-positioned brainstem.
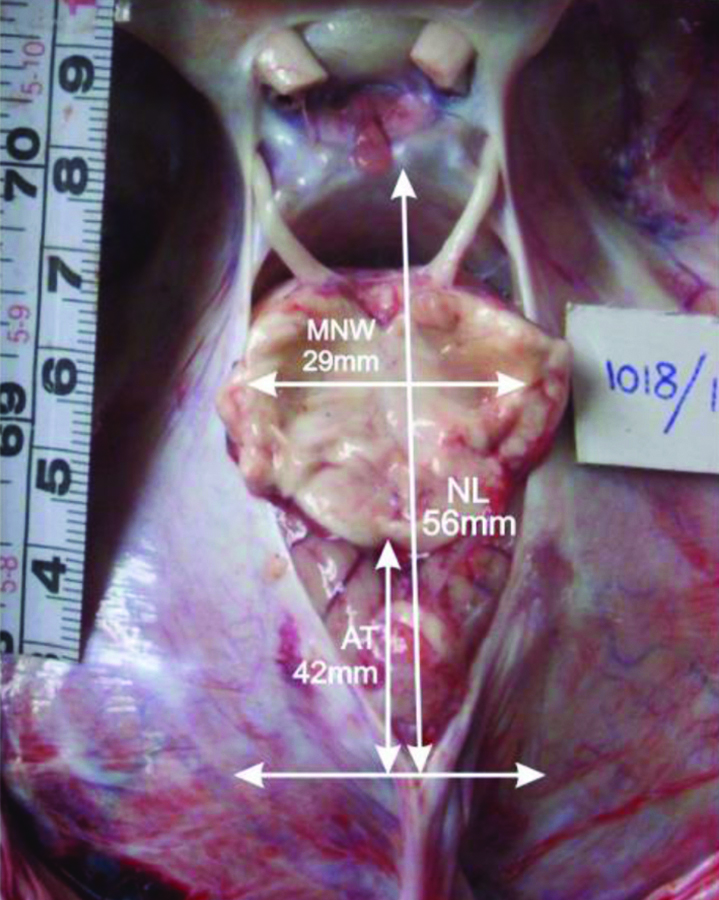
Photograph showing typical type of notch (midrange MNW and midrange NL) and long AT distance exposing greater cerebellar tissue within the notch.
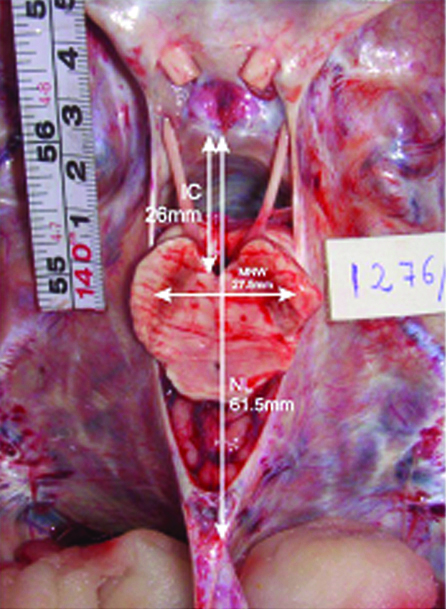
Photograph showing mixed type of notch (narrow MNW and long NL) with a postfixed brainstem. Note the high degree of contiguity between the brainstem and free tentorial edge on both sides.
Photograph showing another type of mixed notch (wide MNW and short NL) and short AT distance with minimal exposure of cerebellar tissue. Note the perimesencephalic space between the brainstem and the free tentorial edge on left side.
Discussion
The anatomical constitution of tentorial notch in present study was compared with that of other populations studied in previous researches [Table/Fig-10]. The observations on ANW and MNW in the present study were similar with previous cadaveric studies [2,6,9]. The wider range of ANW (21 to 34 mm) and MNW (24.5-39 mm) reported by Adler DE and Thomas HM can be attributed to the larger study group (100) [9]. The observations on NL in present study were found similar to that of Adler DE and Thomas HM. Contrastingly, the averages NL reported by Sunderland S in Australian population (54.9±6.93 mm) [6] and by Bull JW in European population (55.3±5.26 mm) were significantly lower statistically significant (p<0.05) [3].
Data comparison of tentorial notch of Indian population with other populations [2,6,9].
| Parameters | Present study(Indian population) | Adler DE and Thomas HM [9](US population) | Michio O et al., [2](US population) | Sunderland S [6](Australian population) |
|---|
| Mean±SD in mm | Range in mm | Mean±SD in mm | Range in mm | Mean±SD in mm | Range in mm | Mean±SD in mm | Range in mm |
|---|
| ANW | 26.92±2.14 | 22-31.5 | 26.6±2.7 | 21-34 | | | 27.06±3.53 | 19-35 |
| MNW | 29.77±2.26 | 24-34 | 29.6±3 | 24.5-39 | 29.6 | 26-35 | 30.16±3.21 | 23-39 |
| NL | 57.98±4.52 | 50-68 | 57.7±5.6 | 44-70 | 52 | 46-67 | 54.9±6.93 | 44-75 |
| IC Distance | 21.21±3.72 | 10-26.5 | 20.4±3.2 | 12-29 | 12.1 | 7.8-15.6 | | |
| AT | 25.81±8.04 | 13-42 | 16.8±5.4 | 4-32 | 19.8 | 13-27 | | |
ANW: Anterior notch width; MNW: Maximum notch width; NL: Notch length, AT: Apicotectal; IC: Interpedunculo-clival; SD: Standard deviation
Sunderland S described a crescent shaped interval anteriorly between the brainstem and the boundaries of the tentorial notch called as ‘tentorial gap’ [6], which roughly corresponds to the anterior incisural space described by Michio O et al., [2]. Dimensions of this gap are important as this is the only unoccupied portion of the notch and brain tissues can herniate freely into this. As it was difficult to measure this three dimensional space, IC distance was measured in present study. The observations regarding IC distance in present study were supported by Adler DE and Thomas HM (20.4±3.2 mm). The study of Michio O et al., needs special mention who observed considerably lower mean IC distance (12.1 mm) and range (7.8-15.6 mm) in comparison to present study. This difference may be attributed to different population ethnicities.
Sunderland S stated that the triangular interval left posteriorly between the midbrain and tentorial margins is occupied by the cerebellar tissue which is closely applied to the tectal surface of the midbrain so that the brainstem is well cushioned from the margins and apex of the notch [6]. This gap corresponds roughly to the posterior incisural space described by Michio O et al., [2]. The amount of cerebellar parenchyma visible through posterior incisural space of the notch varies and is difficult to be quantified. So, AT distance was measured in present study in the axial plane. The mean AT distance (25.81±8.04 mm) in the present study was found to be considerably higher than that reported by Adler DE and Thomas HM (16.8±5.4 mm) and the difference was found to be extremely significant (p-value<0.001). The values observed in present study were also higher than those reported by Michio O et al., (19.8 mm) though it could not be compared statistically due to unavailability of complete data [2]. The range of AT distance (13 to 42 mm) in present study was found higher than those observed by Adler DE and Thomas HM (4-32 mm) and Michio O et al., (13-27 mm) [2]. So, it can be inferred that the AT distance is more in Asian population than that of Western population.
Tentorial notch anatomy and transtentorial herniation: Tentorial herniation is the most common form of brain herniations. The syndrome of transtentorial herniation occurs in wide variety of neurological conditions including haemorrhages, tumours, and brain oedema. Meyer A was the first person to describe the herniation of temporal lobe through the tentorial incisura due to distant effect of supratentorial expanding lesion as a pathological entity [4]. Kernohan JW and Woltman HW described grooving of the cerebral peduncle on the side opposite to the tumour due to indentation of the midbrain against free tentorial edge in some cases of transtentorial herniation [10]. This grooving of cerebral peduncle and the resulting ipsilateral hemiparesis, a false localising sign, were later named as Kernohan’s notch and the Kernohan-Woltman syndrome respectively. Later on, many researchers elucidated the signs and symptoms produced as a result of transtentorial herniation but differing neurological presentations had not been ascribed specific anatomical correlates [11-19]. Despite the clinicopathological understanding of transtentorial herniation, the reasons for unique syndromes that appear among patients had remained ambiguous.
Landmark studies done by Corsellis JA and Sunderland S concluded that the pattern of any herniation must be to some extent affected by the size and shape of the ring through which the brain is being forced [5,6]. Adler DE and Thomas HM formulated the first ever classification system of tentorial notch and presented a hypothesis to explain the difference in manifestations of transtentorial herniations among individuals [9]. It has been shown that large notches contain greater amounts of cerebellar tissue exposed through posterior incisural space in comparison to the small notches [6,9]. Present study also observed a strong positive visual correlation between AT distance and amount of cerebellar tissue exposed and this finding may have implications regarding the propensity for ascending or descending transtentorial herniation. So, present study concur with previous studies that the morphometry of the tentorial notch, together with the location of supratentorial pressures and the rapidity with which they change, play a key role in determining the herniation patterns.
Tentorial notch anatomy and brain injuries: Klintworth GK showed that the tentorium and its aperture remarkably differ in regard to size, shape and orientation among different animal species [20]. The tentorium cerebelli is absent in fishes, reptiles and amphibians whereas it is incomplete in guinea pigs and rodents. These interspecies variations in tentorial anatomy make it difficult to understand the pathophysiological correlates of brain concussion in human beings. Although there is no unique center of consciousness, on the basis of previous experimental studies, it can be postulated that brief and immediate loss of consciousness as a result of brain concussion occurs due to dysfunction of the median raphae and reticular formation in the brainstem [21-27]. In this study, on visual inspection, it was observed that the brainstem was contiguous with the tentorial edges in narrow types of notches. In a narrow notch, the sharp, free tentorial edge may play a direct role in transmission of kinetic energy to the immediately adjacent midbrain [9]. In wide notches, the interval between cerebral peduncles and the tentorial edges facilitates the herniation of uncal tissue and hippocampal gyrus through the aperture on the same side during supratentorial expanding lesions which may cause direct pressure on the oculomotor nerve leading to pupillary dilation [12]. Variations in space between midbrain and clivus (IC distance) may affect the potential for brainstem injury during acceleration-deceleration events. So, present study concur with Adler DE and Thomas HM that anatomical variations in tentorial aperture may affect the clinical sequelae that arise after concussive and inertial brain injuries [9].
The enhancement of these dural structures with the addition of intravenous contrast material permits accurate delineation of the tentorial notch. Authors recommend that the size of the tentorial notch and position of brainstem within it should be determined using modern neuroimaging modalities as it may help in characterising neuro-anatomical variations in this region. These variations in the tentorial aperture may be implicated in a wide spectrum of clinical presentations related to transtentorial herniation, concussion and acceleration-deceleration injuries.
Limitation(s)
During post-mortem examination, it is mandatory to detach the falx cerebri from crista galli so as to remove the forebrain and to measure the morphometric parameters of the tentorial notch. As this study was done during medico-legal autopsies, this may cause unavoidable distortion of normal anatomy of the tentorium. Some anatomical distortion is inherent to a dead brain specimen which can be overcome with the use of neuroimaging techniques to determine tentorial notch morphometry in live human beings.
Conclusion(s)
The present study provides a guideline for the surgeons about the anatomical constitution and variation in the cranium at the level of tentorial notch. Longer NL and AT distance have been observed in this study. This anatomical study may be used to plan the trajectory during neurosurgical operations to be carried out in the vicinity of the tentorial notch and may help in minimising the associated risk and complications. In conclusion, determination of notch type by modern neuroimaging methods, using this anatomical baseline data may influence the treatment of patients with intracranial pathological conditions.
ANW: Anterior notch width, MNW: Maximum notch width, NL: Notch length, IC: Interpedunculoclival, AT: Apico-tectal
PC: Pearson correlation; Sig: Significant two-tailed test; *Correlation is significant at the 0.001 level (2-tailed); ANW: Anterior notch width; MNW: Maximum notch width; NL: Notch length, AT: Apicotectal, IC: Interpedunculoclival
MNW: Maximum notch width; NL: Notch length
MNW: Maximum notch width, NL: Notch length
ANW: Anterior notch width; MNW: Maximum notch width; NL: Notch length, AT: Apicotectal; IC: Interpedunculo-clival; SD: Standard deviation