Postoperative pain is a major concern of majority of patients, and in as many as 30% to 80% it is treated inadequately [1]. This results in harmful acute effects such as adverse physiologic responses and chronic effects like chronic pain and delayed long-term recovery [2]. The optimisation of analgesia can lead to the attenuation of the perioperative pathophysiology and thus reduce complications and facilitate recovery during the immediate postoperative period and after discharge [3].
Breast cancer surgeries are one of the most common cancer surgeries. Modified radical mastectomies are associated with considerable postoperative pain and a combination of oral and intravenous analgesics, in addition to local and regional techniques such as local anaesthetic infiltration, intercostal block, paravertebral block and thoracic epidural anaesthesia are employed to provide analgesia. Though various regional techniques are described for mastectomy, they are not widely used. Opioids remain the most frequently used drug for postoperative analgesia in these patients, which has side-effects like nausea, vomiting, urinary retention, pruritis and respiratory depression that are usually dose related [4,5]. Adjuvants that alleviate postoperative pain and lower the requirement of opioids are often useful in reducing these side-effects.
There are various studies that describe the analgesic potential of beta blockers. They have agreed to the antinociceptive and anxiolytic effects of adrenergic blockade [6-8]. Use of beta blockers perioperatively as an anaesthetic adjunct has been explored in literature [9]. Beta blockers have central action in antinociception and also prolong the duration of action of opioids, thereby decreasing its requirement postoperatively. As beta-adrenergic receptors may potentiate the activity of N-Methyl-D-Aspartate (NMDA) subtype glutamate receptor and facilitate the mechanisms underlying Opioid Induced Hyperalgesia (OIH), beta-adrenergic antagonists are likely to produce antihyperalgesic effects by at least one of these two pathways [10]. Clinically, increase in postoperative opioid requirement in patients receiving opioids is likely due to opioid tolerance or OIH, and as a result it might delay patient’s recovery [11,12]. Therefore, beta blockers may be an effective alternative to counter this.
Esmolol, an ultra-short acting beta blocker was selected for the present study because of its short and transient haemodynamic effects [13]. Intraoperative infusion of esmolol was reported to have postoperative opioid sparing effect by various studies [7,14,15]. But studies pertaining to the use of esmolol in mastectomy is lacking.
The primary objective of the study was to evaluate the analgesic efficacy of esmolol infusion given perioperatively in patients undergoing modified radical mastectomy under general anaesthesia. Numerical Rating Scale (NRS) for pain and requirement of rescue analgesics in the first 24 hour postoperatively were the primary outcome measures. Evaluation of haemodynamic stability and occurrence of side-effects were the secondary objectives.
Materials and Methods
This prospective observational study was done in a Tertiary Care Centre, from June 2017 to August 2018 after getting Institutional Ethics and Research Committee approval (GMCKKD/RP 2017/IEC/130). The study is registered in Clinical trial registry-India (CTRI/2018/05/013571).
Inclusion and Exclusion criteria: This study included 140 patients with carcinoma breast, aged 20-65 years belonging to American Society of Anaesthesiologists’ (ASA) physical status l and ll, who were scheduled for elective modified radical mastectomy under general anaesthesia. Patients with heart disease, bronchial asthma, bradycardia and patients taking beta blockers or analgesic drugs regularly for other reasons were excluded from the study.
They were divided into two groups of 70 each, group E (esmolol) and group C (saline). Sample size calculation was done using formula; n=[(za+zb)2×SD2×2]/d2. Standard deviation for NRS was 1.4 as per study by Bhawna SJ et al., [8]. To detect a difference of NRS score 0.67 between the two groups with power of study 80% and alpha at 5%, sample size required was 70 in each group. Preoperatively, patients were familiarised to the NRS of pain assessment, and were taught how to respond according to their pain status in the postoperative period when enquired. NRS for pain was assessed as-no pain (0), mild-(NRS-1-3), moderate-(NRS 4-6) and severe-(NRS-10) during the first 24 hour postsurgery.
The dose of esmolol was chosen so as to obtain its analgesic effect with minimal side-effects, i.e., 0.25 mg/kg bolus dose and intra operative infusion of 5 μg/kg/min [16]. On the morning of surgery, all patients were taken to the premedication room and baseline parameters including Heart Rate (HR), Mean Arterial Pressure (MAP) and SpO2 were noted. After psychological preparation and explanation of the procedure to the patient, an intravenous line was established on the arm opposite to the side of surgery under local anaesthesia.
An anaesthesiologist in the premedication room allotted patients into one of the two groups using computer generated random number chart and set the drug for each patient. He maintained a chart of the drug and the patient to which the attending anaesthesiologist had no access. The aforesaid anaesthesiologist did not take part in intraoperative or postoperative monitoring or statistical analysis. The drug was labeled with the name of the patient only. Hence, both the patient and the assessor were unaware of the allotted group and drug.
All patients were premedicated with midazolam 0.02 mg/kg, glycopyrrolate 0.2 mg, ondansetron 0.1 mg/kg and morphine 0.1 mg/kg intravenously and taken to the operation theatre. Another intravenous line was secured in the lower limb under local anaesthesia for the infusions. Ten minutes before induction, patients in esmolol group received a bolus dose of 0.25 mg/kg esmolol diluted in 10 mL saline, given over a period of ten minutes, followed by a continuous infusion of esmolol 5 μg/kg/min until the completion of surgery using an infusion pump. Patients in group C received equivalent volumes of saline as bolus and infusion.
All patients were induced with titrated doses of propofol till loss of response to call. Endotracheal intubation was done with appropriate size cuffed endotracheal tube facilitated by succinyl choline 2 mg/kg. Anaesthesia was maintained with oxygen, nitrous oxide, propofol infusion (50 μg /kg /min) and vecuronium.
Intraoperative HR, MAP and SpO2 were monitored and recorded, initially at three-minute intervals till 30 minutes and then at five-minute intervals. An increase in heart rate >20% above baseline or increase in MAP >20% above baseline for more than one minute and presence of autonomic signs (lacrimation, sweating) were assessed by the attending anaesthesiologist and considered as inadequate anaesthesia and appropriate adjustment of propofol infusion was done. Intraoperative hypotension defined as MAP <60 mmHg or if HR is less than 50/min, adjustment of esmolol infusion was done. Continuous monitoring of haemodynamic parameters, HR, MAP and SpO2 were ensured to detect the occurrence of adverse effects like bradycardia and hypotension which were managed appropriately, either by drugs, or by reducing the infusion rate of esmolol.
Propofol infusion was discontinued at the start of skin closure. Local infiltration of 15 mL of 0.25% bupivacaine was given at the incision site for both the groups at the end of the surgery. Esmolol or saline infusion was discontinued at the end of the surgery. Duration of surgery was noted in all cases. Patients were reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg).
Postoperative pain was recorded by another anaethesiologist who was completely unaware of the test and control groups. This eliminated the bias. Patients were asked to indicate the strength of pain according to the NRS scale. Tramadol 50 mg intravenously was administered on patient demand irrespective of the NRS score and the time to the first dose was noted. Up to 2 mg/kg of tramadol was given intravenously if pain was not relieved. Total dose of tramadol was restricted to 300 mg in 24 hour. Total number of bolus doses of tramadol administered and its total consumption for first 24 hour was noted. All these were recorded at 2, 4, 6, 8, 12 and 24 hours. Additional doses of ondansetron were given on complaints of nausea and vomiting due to tramadol injection. Occurrence of any other side-effects were also looked out for and recorded during this period.
STATISTICAL ANALYSIS
Descriptive statistics of numerical rating scores and analgesic requirements were analysed in terms of mean and standard deviation with help of Statistical Package for Social Sciences (SPSS) statistics version 18. Independent t test was used to compare numerical rating scores and analgesic requirement of the two groups. A p-value of <0.05 was considered statistically significant.
RESULTS
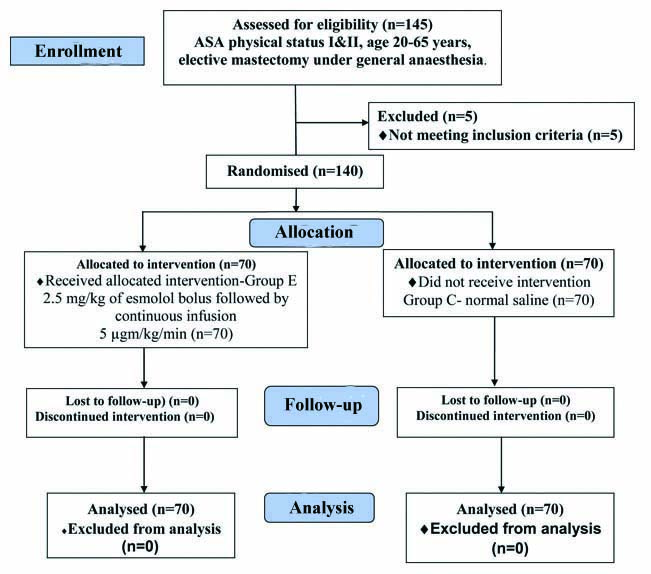
A total of 140 patients, 70 in each group belonging to ASA I and II were included in the study [Table/Fig-1]. Both the groups were comparable in terms of demographic variables like age, weight, ASA status and also for duration of surgery [Table/Fig-2].
Flow chart representing study participants.
Comparison of demographic variables.
| Parameter | Group E | Group C | p-value |
|---|
| Age (Years) | 52.87±3.897 | 52.26±4.282 | 0.376* |
| Weight (kg) | 54.27±5.929 | 54.07±5.145 | 0.832* |
| ASA I | 47 | 51 | 0.461† |
| ASA II | 23 | 19 |
| Duration of surgery (min) | 64.67±14.507 | 65.86±14.471 | 0.662* |
*Independent sample t-test; †Chi-square test; ASA: American society of anaesthesiologists; p-value <0.05 considered significant
There was significant difference in mean NRS between two groups with lower mean NRS over time in the esmolol group. The p-value at all monitored time frames were less than 0.05. The time to first rescue analgesic dose was prolonged significantly in esmolol group with p-value of <0.001 [Table/Fig-3].
Comparison of numerical rating scale of pain.
| Parameter | Group E | Group C | *p-value |
|---|
| NRS-2 hr | 0.27±0.588 | 2.57±1.084 | <0.001 |
| NRS-4 hr | 0.44±0.754 | 3.76±1.096 | <0.001 |
| NRS-6 hr | 0.64±0.979 | 4.83±1.251 | <0.001 |
| NRS-8 hr | 1.17±1.251 | 5.66±1.075 | <0.001 |
| NRS-12 hr | 1.97±1.383 | 6.04±0.999 | <0.001 |
| NRS-24 hr | 2.69±1.325 | 6.49±0.944 | <0.001 |
| Time to first rescue analgesic dose | 17.59±5.012 | 8.21±2.226 | <0.001 |
*Independent sample t-test; p-value <0.05 statistically significant; NRS: Numerical rating scale
In esmolol group, 18 patients were comfortable without any additional analgesic for the first 24 hour. Forty five patients had a single dose of rescue tramadol administration whereas in the control group, all patients needed rescue analgesic and only five were satisfied with single dose. The total mean requirement of tramadol in 24 hour was 42.14±29.03 mg in esmolol group whereas that of control group was 102.86±22.3 mg [Table/Fig-4].
Comparison of analgesic requirement between the two groups.
| No. of analgesic doses | Group E n (%) | Group C n (%) |
|---|
| 0 | 18 (25.7) | 0 (0) |
| 1 | 45 (64.2) | 5 (7.1) |
| 2 | 7 (10) | 56 (80) |
| 3 | 0 | 9 (12.9) |
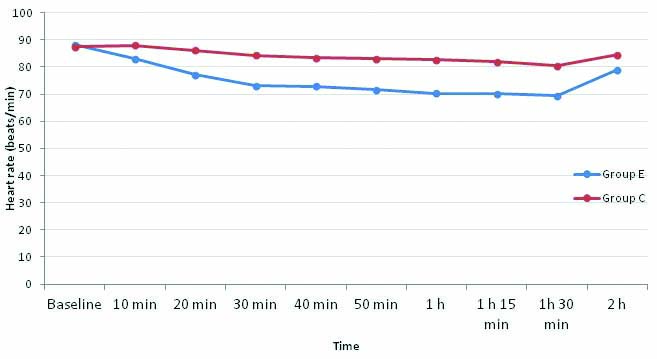
Baseline heart rates were comparable as the p-value was 0.669. The mean heart rate in the control group remained similar to baseline at the time of induction (10 min after start of infusion) whereas in the esmolol group, the mean HR was lower than baseline. Though the heart rate in the esmolol group continued to be statistically lower throughout the intraoperative period, none of the patients went into bradycardia or needed any interventions [Table/Fig-5].
Comparison of mean heart rates of two groups.
p-value <0.05 statistically significant; Independent sample t-test. p-value 0 h: 0.669, 10 min: <0.010, 20 min: <0.001, 30 min: <0.001, 40 min: <0.001, 50 min: < 0.001, 1 h: <0.001, 1 h 15 min: <0.001; 1h 30 min: <0.001; 2h: <0.001
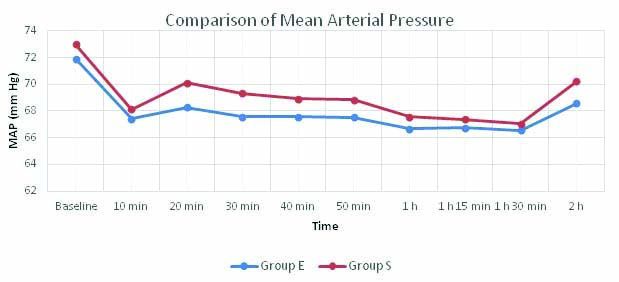
The MAP of the two groups remained lower than baseline at all monitored times. Esmolol group had a lower MAP than control group but all values were within 20% of baseline and the difference was not significant between the groups before infusion, intraoperatively and at all other time frames till postsurgery [Table/Fig-6].
Comparison of MAP between the two groups.
p-value <0.05 statistically significant; Independent sample t-test; p-value 0 h: 0.191, 10 min: 0.430, 20 min: 0.053, 30 min: 0.073, 40 min: 0.128, 50 min: 0.156, 1 h: 0.319, 1 h 15 min: 0.442; 1h 30 min: 0.572; 2h: 0.53
Discussion
About 60% of postmastectomy patients continue to experience chronic pain even after nine months and acute postoperative pain has been implicated as an important predictive factor [17]. Variety of measures has been employed to reduce acute postoperative pain in mastectomy. These include various regional techniques and systemic opioids and non-opioid analgesics [18,19]. Opioid-free anaesthesia is the new gospel in malignancy surgeries. Many drugs like dexmedetomidine, gabapentin, lignocaine, magnesium, clonidine etc., have been tried as anaesthetic adjuvants for decreasing the opioid requirements and its related side-effects [20-22]. There are several studies that quote the analgesic efficacy of esmolol infusion in various surgical procedures, but not many in mastectomy [8,23]. In the present study, esmolol 0.25 mg/kg bolus administered ten minutes before induction and continued as an infusion (5 μg/kg/min) till the end of surgery as an adjuvant to morphine was studied and observed significantly better analgesic profile in terms of lower NRS scores and reduced rescue analgesic requirements than saline placebo.
The use of various adjuvants has been researched in acute postmastectomy pain. Dexmedetomidine as adjuvant was used in breast surgeries in studies by Jain G et al., and Kim SH et al., as 24-hour continuous infusion and single bolus dose respectively [22,24]. Though the Visual Analogue Score (VAS) scores were similar to placebo group, lower analgesic requirement was observed with single bolus administration whereas both lower pain scores (verbal numerical scale) and lower analgesic requirement was observed with 24-hour continuous infusion of dexmedetomidine. In this study, similar results with lower NRS scores and less rescue analgesic requirement could be observed even without continuing esmolol infusion postoperatively. In the esmolol group, the time to first demand of rescue analgesic was prolonged and 25% patients had no demand for rescue analgesic at all in the first 24 hours, whereas in the placebo group all participants required tramadol and more than 80% patients consumed multiple doses. Gosai N et al., who compared preoperative oral gabapentin, oral clonidine and placebo in modified radical mastectomy documented substantial reduction of pain (VAS max 3.6 for gabapentin and 4.8 for clonidine) in both groups compared to placebo with more than 50% reduction in rescue analgesic (diclofenac) requirements in the postoperative period [21]. In the present study the total mean requirement of tramadol in 24 hour was 42.14±29.03 mg in esmolol group which was less than 50% of what was required in the control group (102.86±22.3 mg) (p<0.0001).
The results in the present study were consistent with those of previous studies that used esmolol infusion perioperatively [7,8,23]. In all these studies, there was significant reduction in requirement of opioids postoperatively. In a recent meta-analysis of studies comparing different doses of esmolol bolus and infusion for laparoscopic surgeries, abdominal surgeries, septorhinoplasty etc., by Gelineau AM et al., it was proved that intraoperative esmolol reduces both intraoperative and postoperative opioid requirement [23]. Bhawna SJ et al., in their study comparing esmolol and placebo in lower abdominal surgeries also came to inference that perioperative esmolol infusion decreased postoperative morphine consumption [8]. Chia YY et al., administered 0.5 mg/kg of esmolol bolus followed by infusion of 0.05 mg/kg/min before induction in abdominal hysterectomy and concluded that there was reduction in the intraoperative inhalation anaesthetic and fentanyl usage, stable haemodynamics and reduced morphine consumption for the first three postoperative days [7]. Kavak Akelma F et al., found a better opioid sparing effect of esmolol over lignocaine infusion for postoperative analgesia in laparoscopic cholecystectomy patients [25].
Esmolol, by virtue of its beta blocking property produces lowering of heart rate. The heart rate in the esmolol group was significantly lower than the control group, but was not clinically significant to produce bradycardia requiring intervention. The lowest heart rate recorded was 50 in esmolol group, but was transient and no treatment was required. A systematic review of the safety on perioperative esmolol by Landoni G et al., demonstrated no significant increase in bradycardia or hypotension in non-cardiac surgeries where as another study by Lopez-Álvarez S et al., had two episodes of bradycardia requiring treatment [16,26].
In a meta-analysis, Yu SK et al., stated that hypotension with esmolol was dose-related and was associated with a fixed dosing schedule rather than titrating to HR and blood pressure [27]. Low initial bolus doses of esmolol, with a continuous infusion strategy, resulted in fewer episodes of hypotension. This report documents that titration of esmolol can achieve a targeted reduction in both heart rate and blood pressure. In the present study, the MAPs in both esmolol and control groups were within 20% of the baseline values, not showing a statistically significant difference. This showed that there were no major haemodynamic variations while using the drug as intraoperative infusion. This maybe because of low dose esmolol used as bolus and continuous infusion in this study.
There were no episodes of nausea or vomiting in both the groups in this study. This may be probably due to the prophylactic use of intravenous ondansetron in both the groups. Moon YE et al., also had similar results and attributed it to prophylactic ondansetron [28]. In a meta-analysis to compare esmolol and opioids on postoperative nausea and vomiting, Thiruvenkatarajan V et al., assessed six studies in laparoscopic surgeries and reduction in the incidence was noted in patients who received esmolol compared to those who received opioids [29]. Prophylactic antiemetics such as dexamethasone, droperidol and ondansetron were administered alone and in combinations in three of these studies while the remaining three studies did not report the use of prophylactic antiemetics.
Limitation(s)
The present study was conducted in a small study population and included only females. Bispectral index monitoring was not used and hence, evaluation of the effect of esmolol on intraoperative anaesthetic requirements was not done. Esmolol may have masked the increase in heart rate and blood pressure due to its beta blocking activity. Future studies can be done to evaluate the effects for longer periods (72 h) and reduction of chronic pain after mastectomy which is a major problem that affects the quality of life of these patients.
Conclusion(s)
Perioperative esmolol infusion when used as an adjunct to morphine in patients undergoing modified radical mastectomy significantly decreased postoperative pain and analgesic requirements without any haemodynamic instability.
*Independent sample t-test; †Chi-square test; ASA: American society of anaesthesiologists; p-value <0.05 considered significant
*Independent sample t-test; p-value <0.05 statistically significant; NRS: Numerical rating scale