Gastro-duodenal disorders are a frequent cause of clinical disease, with inflammatory and neoplastic lesions being particularly common. H.Pylori chronically infects more than half of the world’s population and the most prolific etiological agent responsible for various gastro-duodenal lesions like acid peptic disease and sometime malignant lesions. Thus, there is great interest in its detection and eradication. There are various methods to detect H.Pylori-noninvasive tests like rapid urease test, stool antigen test and invasive tests like biopsy using stains like H&E, Giemsa and IHC. With IHC, coccoid forms of H. pylori can be distinguished from other bacterial cocci, fungal spores, and cryptosporidia that may colonise the human stomach [1,2].
The timely and accurate diagnosis of gastric cancer is pivotal as the 5-year survival rate of Early Gastric Cancer (EGC) and Advanced Gastric Cancer (AGC) is approximately 90% and 30%, respectively. Endoscopic visualisation and mucosal biopsy are the investigative tools of choice for diagnosis of gastric cancer [3]. Current population-based studies recorded, the incidence rate of gastric cancer in patients with (gastric) Intestinal Metaplasia (IM) was about 0.38 to 1.29 per 1000 person years. In the era of therapeutic endoscopy, surveillance of patients with IM is necessary to detect gastric malignancy in the early stage, so as to reduce morbidity and mortality of patients [4]. As H.pylori has been widely implicated in pathogenesis of various inflammatory lesions and carcinogenesis, its detection is of utmost importance in appropriate patient care. Routine H&E and Giemsa staining methods can sometimes give false negative results. Therefore, the present study proposes the utility of immunohistochemical method using H.pylori antibody to detect the bacteria.
Materials and Methods
The present observational study was conducted at the Pathology Department, in collaboration with Department of Gastroenterology in a tertiary care hospital in Kalaburagi, Karnataka over a period of 5 years (1 July 2014 to 30 June 2019, 3 years retrospective and 2 years prospective). Study sample included all endoscopic gastric biopsies received during the study duration.
Total of 95 cases were studied. Reviewing retrospective cases, approximately sample size was assessed. Sample size was calculated using formula: Sample size(n)=4pq/L2 All patients referred to endoscopic section, were clinically examined and complete history was documented. For the retrospective cases, clinical details were obtained from records. Blocks were retrieved for the special stains and IHC and the histopathology slides were reviewed. Ethical Committee Clearance was obtained to carry-out the study (IEC no:191003).
Inclusion criteria: The samples include all gastric biopsies which are done for various chronic upper abdominal symptoms like abdominal pain, dyspepsia, heartburn, nausea, vomiting and also for associated systemic manifestations like anorexia, weight loss etc.
Exclusion criteria: Biopsies from oesophagus, autolysed specimens and crushing artifacts were excluded.
Biopsies were fixed in 10% formalin and histopathological slides prepared for staining with H&E, Giemsa and Immunohistochemical methods (Anti-H. Pylori, mouse monoclonal antibody in PBS with carrier protein: BioGenex) using paraffin blocks. Grading of cancerous lesions was done according to WHO classification (2019) [5]. Sydney system of reporting was used for classifying gastritis [6].
Statistical Analysis
In the present study, a total of 95 endoscopic biopsies from July 2014 to June 2019 were studied, categorisation and tabulation of various gastric lesions encountered were compared with the previous studies.
Results
The age ranged from 19 to 80 years with a mean age of 49.75 years. Most of the cases were observed in the age group 51-60 years and most of them were male (70 cases) with a male to female ratio of 2.8:1. Most common indication for endoscopic biopsy was dyspepsia 77 (71.29%) followed by vomiting 24 (22.22%).
Majority of the benign cases were CAG 23 (24.21%) followed by CSG [Table/Fig-1] 14 (14.73%) and among malignant cases adenocarcinoma was predominant 21 (22.10%) [Table/Fig-2].
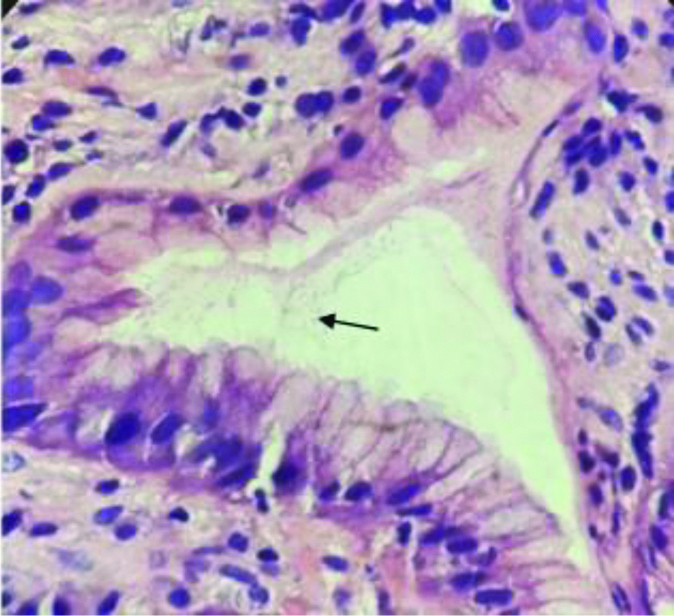
Chronic Superficial Gastritis (CSG) showing gastric epithelium magenta pink and Intestinal Metaplasia (IM) staining blue. (10x, Alcian blue-PAS stain)
PAS: periodic acid Schiff
Distribution of histomorphological lesions and H.Pylori.
| Histomorphological lesions | No of cases | Percentage (%) n=95 | H.Pylori staining (IHC) |
|---|
| Chronic Active Gastritis (CAG) | 23 | 24.21 | 16 |
| Chronic Superficial Gastritis (CSG) | 14 | 14.73 | - |
| Lymphocytic gastritis | 09 | 9.47 | 03 |
| Gastric ulcer | 08 | 8.42 | - |
| Chronic atrophic gastritis | 07 | 7.36 | - |
| Acute erosive gastritis | 04 | 4.21 | - |
| CAG with Intestinal Metaplasia (IM) | 04 | 4.21 | - |
| CSG with Intestinal Metaplasia (IM) | 03 | 3.15 | 01 |
| Adenocarcinoma | 21 | 22.10 | - |
| Non-Hodgkin Lymphoma (NHL) | 02 | 2.10 | 01 |
| Total | 95 | 100 | 21 |
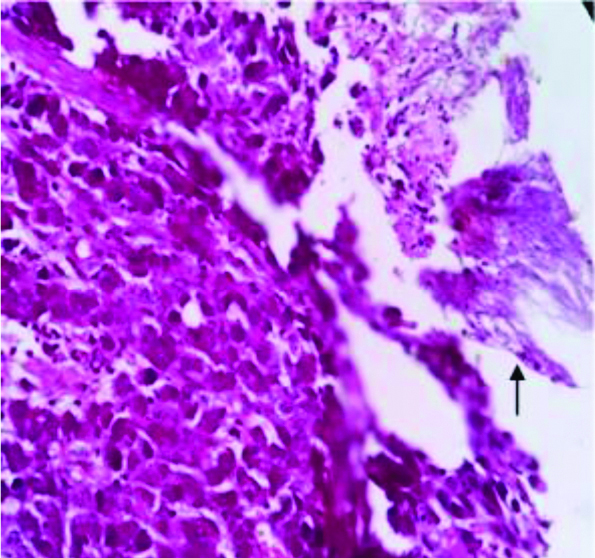
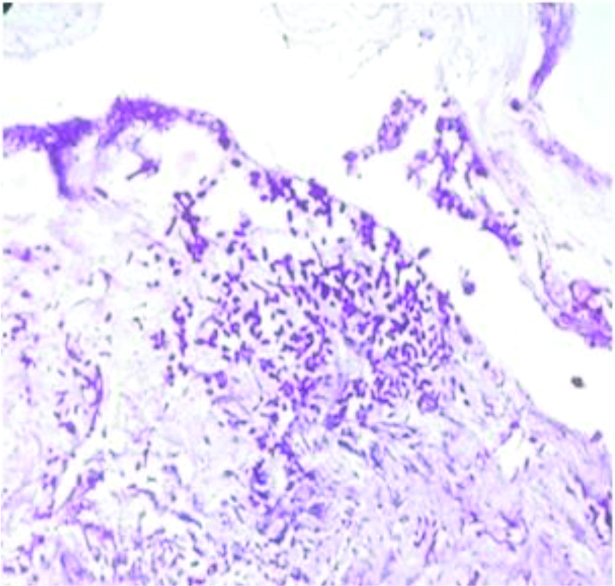
In the present study, moderately differentiated and poorly differentiated adenocarcinoma 9 (39.13%) accounted majority of the cases among the malignant tumours [Table/Fig-3]. Mean age of patients with malignant lesions was 55years. Male to female ratio was 1.9:1. Candidiasis was identified in one case of poorly differentiated adenocarcinoma case [Table/Fig-4] and the same was demonstrated by PAS stain [Table/Fig-5].
Distribution of gastric malignant tumours.
| Malignant tumours | No of cases | Percentage (%) |
|---|
| Adenocarcinoma-Well differentiated-Moderately differentiated-Poorly differentiated | 21 | 91.30 |
| 03 | 13.04 |
| 09 | 39.13 |
| 09 | 39.13 |
| Non-Hodgkin lymphoma (NHL) | 02 | 8.69 |
| Total | 23 | 100 |
Poorly differentiated adenocarcinoma with candidal hyphae (arrow). (40x, H&E stain)
H&E: Haematoxylin and Eosin
Magenta pink candida spores (periodic acid schiff stain, 40x).
Most common lesion was CAG, in which H.Pylori were identified by H&E [Table/Fig-6], Giemsa [Table/Fig-7] and IHC [Table/Fig-8]. The staining by only IHC was positive in one case each of CSG with IM and Non-Hodgkin Lymphoma (NHL) [Table/Fig-9,10] only. CD45 was done to confirm NHL [Table/Fig-11a,b,12].
Spiral shaped H.pylori in the gastric luminal epithelium (40x, H&E stain).
H.Pylori in the luminal epithelium in Chronic Active Gastritis (CAG). Giemsa (40x)
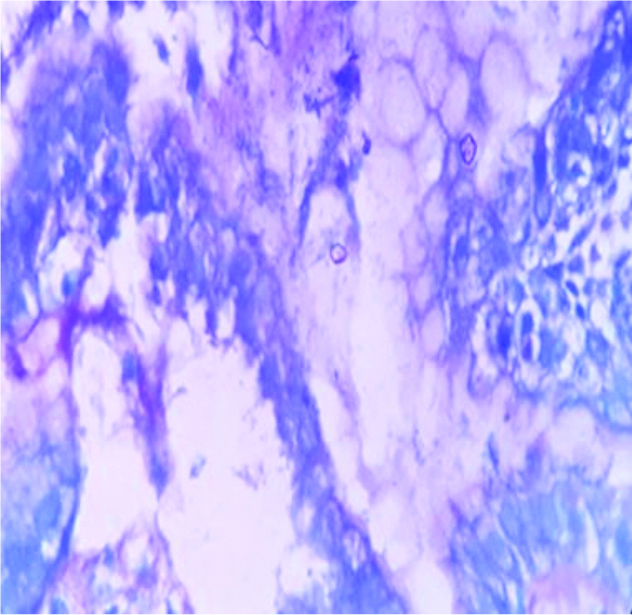
H.Pylori in the luminal epithelium in Chronic Active Gastritis (CAG). IHC (10x)
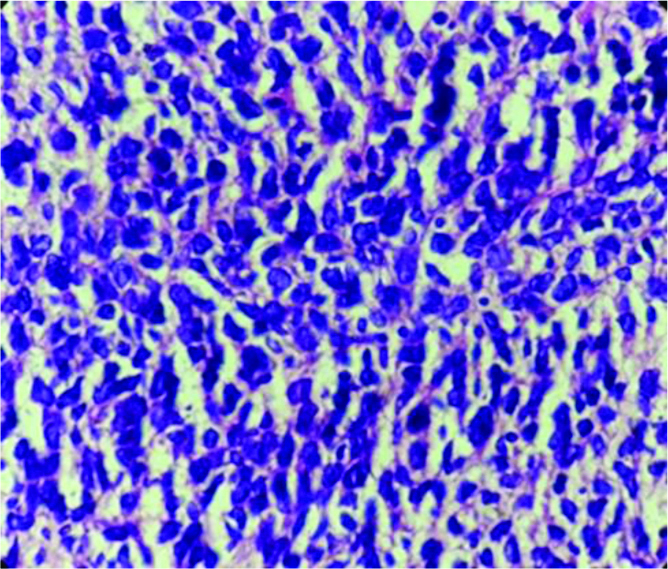
Diffuse sheets of monotonous population of lymphoid cells-Non-Hodgkin Lymphoma (NHL) (40x) H&E.
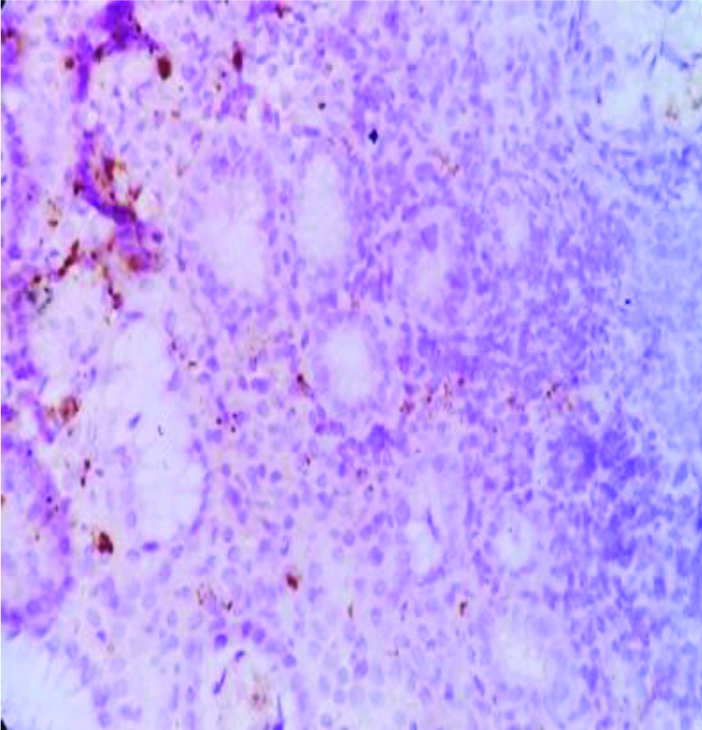
NHL - H. pylori positive in glandular epithelium. IHC(10x)
NHL-CD 45 positive of lymphoma cells, adjacent epithelium is negative. IHC(10x)
NHL-CD 45 positive of lymphoma cells (40x) IHC.
IHC: Immnohistochemical; NHL: Non hodgkin lymphoma

H.Pylori staining in comparison to other stains.
| Lesions (n=Total no of cases) | H&E | Giemsa | IHC |
|---|
| Chronic Active Gastritis (CAG) (23) | 11 | 13 | 16 |
| Lymphocytic gastritis (09) | 01 | 01 | 03 |
| Chronic Superficial Gastritis (CSG) with IM (03) | 00 | 00 | 01 |
| Non-Hodgkin Lymphoma (NHL) (02) | 00 | 00 | 01 |
| Total (n=95) | 12 | 14 | 21 |
IHC was considered the gold standard method for detection of H.pylori. Sensitivity of H&E and Giemsa stain was 57.14% and 66.67%, respectively and specificity was 100% for both stains. PPV was 100% for both satins and NPV was 89.16% for H&E, and 91.36% for Giemsa stain.
Discussion
The gastric diseases have wide spectrum of inflammatory lesions such as gastritis and acid peptic disease to frankly malignant such as gastric carcinoma and lymphoma. Of the diverse etiological associations, the most important and the most common infection worldwide is a bacterium named H.Pylori which has a vital role in causation of gastritis, metaplastic change, adenocarcinoma sequence and lymphoma [7].
H.Pylori is classified as a carcinogen, and about half of the world population is infected with H.Pylori, with the burden of the disease highest in developing countries like India. The infection is related to overcrowding and poor hygiene. The probable mode of transmission is by person to person contact between the family members and also through contaminated food and water [8].
The causative role of H.pylori in the development of chronic gastritis is not only associated with bacteria but also to host and environmental factors. Assessing risks for gastric cancer can be achieved by the evaluation of clinical, morphological and molecular factors. Among morphological criteria, atrophy and IM can be evaluated using Sydney and OLGA approaches for unbiased diagnosis [9].
In gastro-intestinal tract candidiasis, following oesophagus, stomach and intestines are the uncommon sites involved. Gastric candidiasis is seen with altered gastric mucosal integrity in benign and malignant gastric ulcers, intake of corrosive chemicals, postsurgery, gastric perforation. Its association with benign gastric ulcers is frequent compared to malignant ulcers [10].
Gastric carcinogenesis is heterogeneous and extremely diverse at the molecular level. Nonetheless the gene errors are plentiful, no specific or comprehensive molecular markers, gene errors or mutations have been found in cancer or in pre-cancerous conditions so far. Although the two main histological types of gastric cancer, intestinal and diffuse, and both being bound to a preceding or co-existing H.pylori gastritis, the types may not be fully distinct entities. In diffuse type, the background morphology is often a non-atrophic H.pylori gastritis, indicating that the carcinogenetic mechanisms are linked with the inflammation. In intestinal cancer type, on the other hand, associated often with a hypochlorhydric stomach, and with a marked atrophic gastritis, and with extensive IM in the underlying mucosa [11].
Chiang TH et al., study reveals mass screening and eradication of H.Pylori infection offer greatest benefit in regions with high gastric cancer risk which reduce gastric cancer incidence, without evidence of adverse consequence [12]. New studies also highlight that microRNA-mediated regulation and epigenetic modifications, through DNA methylation, play major role in H.Pylori-induced tumourigenesis process [13].
Histomorphological Lesions
CAG was commonest lesion in present study (24.21%) and which correlated with Mithun V et al., study (33.63%) [Table/Fig-13] [1].
Comparative analysis of Histomorphological lesions.
| Histomorphological lesions | Sulakshana MS et al., [14] 2015 | Mithun V et al., [1] 2016 | Present study 2019 (%) |
|---|
| Chronic Active Gastritis (CAG) | 08 (8) | 74 (36.27) | 23 (24.21) |
| Chronic Superficial Gastritis (CSG) | 12 (12) | 80 (39.21) | 14 (14.73) |
| Lymphocytic gastritis | - | - | 09 (9.47) |
| Gastric ulcer | 14 (14) | - | 08 (8.42) |
| Chronic atrophic gastritis | - | - | 07 (7.36) |
| Acute erosive gastritis | - | 16 (7.84) | 04 (4.21) |
| CAG with Intestinal Metaplasia (IM) | - | 09 (4.41) | 04 (4.21) |
| CSG with Intestinal Metaplasia (IM) | - | 12 (5.82) | 03 (3.15) |
| Adenocarcinoma | 48 (48) | 08 (3.92) | 21 (22.10) |
| Non-Hodgkin Lymphoma (NHL) | 01 (1) | - | 02 (2.10) |
| Others | 17 (17) | 05 (2.45) | - |
| Total | 100 (100) | 204 (100) | 95 (100) |
In present study, adenocarcinoma was the most common type of malignant tumour, constituting 91%. Similar high predominance of adenocarcinoma was also reported by Sulakshana MS et al., and Hussain SI et al., [14,15]. In present study majority of adenocarcinoma were of moderately and poorly differentiating subtypes. Similar observation was also made by Sulakshana MS et al., [14]. NHL was the least common variety in present study [Table/Fig-14].
Comparative analysis of Gastric malignant tumours.
| Malignant tumours | Hussain SI et al., [15] (%) n=39 | Sulakshana MS et al., [14] (%) n=50 | Present study (%) n=23 |
|---|
| Adenocarcinoma | 39 (100) | 48 (96) | 21 (91.30) |
| Well differentiated | 10 (25.64) | 10 (20) | 03 (13.04) |
| Moderately differentiated | 20 (51.28) | 15 (30) | 09 (39.13) |
| Poorly differentiated | 09 (23.07) | 18 (36) | 09 (39.13) |
| NHL (Non-Hodgkin Lymphoma) | - | 01 (02) | 02 (8.69) |
| Others | - | 06 (12) | - |
| Total | 39 (100) | 50 (100) | 23 (100) |
Staining for H Pylori Organism
In present study, H pylori was detected in 22.10% (n=95) of all samples, which was lesser compared with studies by Patnayak R et al., Kacar F et al., Dogar T et al., Bamanikar S et al., and Sultana A et al., [16-20]. Heterogeneity of cases and inclusion of retrospective samples and other confounding factors like use of Proton Pump Inhibitors (PPI) might have resulted in lower detection rates [Table/Fig-15].
Comparative analysis of H. Pylori staining.
| Study authors (n-No of cases) | Year of publication | H&E | Giemsa | IHC |
|---|
| Kacar F et al., (70) [17] | 2003 | 58 (82.85%) | 58 (82.85%) | 60 (85.71%) |
| Sultana A et al., (100) [20] | 2011 | 25 (23.80%) | 27 (25.71%) | - |
| Dogar T et al., (70) [18] | 2012 | 19 (27.2%) | - | 23 (32.85%) |
| Patnayak R et al., (79) [16] | 2015 | 26 (32.9%) | 26 (32.9%) | 49 (62%) |
| Bamanikar S et al., (985) [19] | 2018 | 397 (40.30%) | 484 (49.14%) | 519 (52.69%) |
| Present study (95) | - | 12 (12.63%) | 14 (14.73%) | 21 (22.10%) |
In the present study, sensitivity of H&E and Giemsa stain was 57.14% and 66.67%, respectively and specificity was 100% for both stains. PPV was 100% for both stain and NPV was 89.16% for H&E, and 91.36% for Giemsa stain. Shukla S et al., study revealed sensitivity, specificity, PPV and NPV of H&E and modified Giemsa stain were 72.5%, 80.4% and 100%, 100%, 100%, 100% and 78.5% and 83.6%, respectively [21]. In present study, sensitivity and specificity of H&E and Giemsa stain was lower compared to Shukla S et al., study [21]. Presence of inflammation, lower density of H pylori is known to affect detection rate by H&E method. We presume older specimen also affects staining characteristics. The present study show less number of H.pylori bacilli not detected by H&E and Giemsa which were detected by IHC staining. There were no false negative cases in the present study [Table/Fig-16].
Comparative analysis of H&E and Giemsa staining performance.
| Staining method | H&E | Giemsa |
|---|
| Comparative analysis | Shukla S et al., [21] | Present study | Shukla S et al., [21] | Present study |
|---|
| Sensitivity | 72.5% | 57.14% | 80.4% | 66.67% |
| Specificity | 100% | 100% | 100% | 100% |
| PPV | 100% | 100% | 100% | 100% |
| NPV | 78.5% | 89.16% | 83.6% | 91.36% |
H&E: Haematoxylin and Eosin; PPV: Positive predictive value; NPV: Negative predicve value
Mass screening and eradication of H.Pylori infection can effectively reduce the gastric malignancy incidence, significantly lessen the number of related deaths and reach the goal of eliminating the gastric malignancy.
Limitation(s)
The demonstration of this bacteria by IHC can be gold standard method; however, in the resource limited settings, routine use of IHC may not be cost-effective. The study needs to be conducted on large population to arrive at statistically significant results.
Conclusion(s)
Advancement in the diagnostic procedures of gastric lesions has resulted in the better diagnosis and effective planning of the management. We conclude that the routine H&E and Giemsa staining methods can reliably help in detection of H.Pylori in various gastric lesions; whereas demonstration of bacteria using immunohistochemical staining has been more promising compared to the routine stains and can be considered as the gold standard method. However, in developing countries like India, utilisation of IHC routinely may not be cost-effective, but can be considered in cases with ambiguity in routine H&E and Giemsa results.