Non-typhoidal Salmonella (NTS) are generally associated with self-limiting gastrointestinal disease, often acquired through the ingestion of contaminated food and it seldom requires antimicrobial therapy for treatment. Extra-intestinal manifestations could be localised infection leading to septic arthritis, osteomyelitis. In complicated invasive disease, there could be bronchopneumonia with or without bacteraemia leading to mortality. Invasive NTS infections are infrequently reported in India. The S. Typhimurium is one of the common serovars associated with invasive disease and its virulence factors are responsible for causing the disease. S. enteridies, S. Dublin are the other serovars which are commonly responsible for invasive NTS infection. It is difficult to diagnose invasive disease without appropriate bacteriological culture based method. With emergence to resistance to antimicrobials the treatment of this condition is also becoming challenging. In this case report, a five-month-old infant presented with cough fever, stuffed nose dyspnoea and was diagnosed as bronchopneumonia. Mechanical ventilation was required for five days along with admission to intensive care unit. Invasive NTS infection was diagnosed using automated blood culture and the child responded to intravenous antimicrobial chemotherapy.
Blood culture, Bronchopneumonia, Food borne infection, Salmonella Typhimurium
Case Report
A five-month-old male child was brought to the Paediatrics OPD by his parents. They complained that the child had cough along with nasal stuffiness for past six days which was followed by onset of fever and rapid breathing in last three days. The child was admitted with a high-grade intermittent fever of 102°F, dyspnoea (74 breaths/min), tachypnoea (152 beats/min). The child had received syrup containing amoxicillin clavulanic acid for three days prior to admission.
The baby was born at term via normal vaginal delivery. The APGAR score was normal after birth and there was no untoward complications prior to or at the time of delivery. The mother was feeding the child with diluted cow’s milk for past one month.
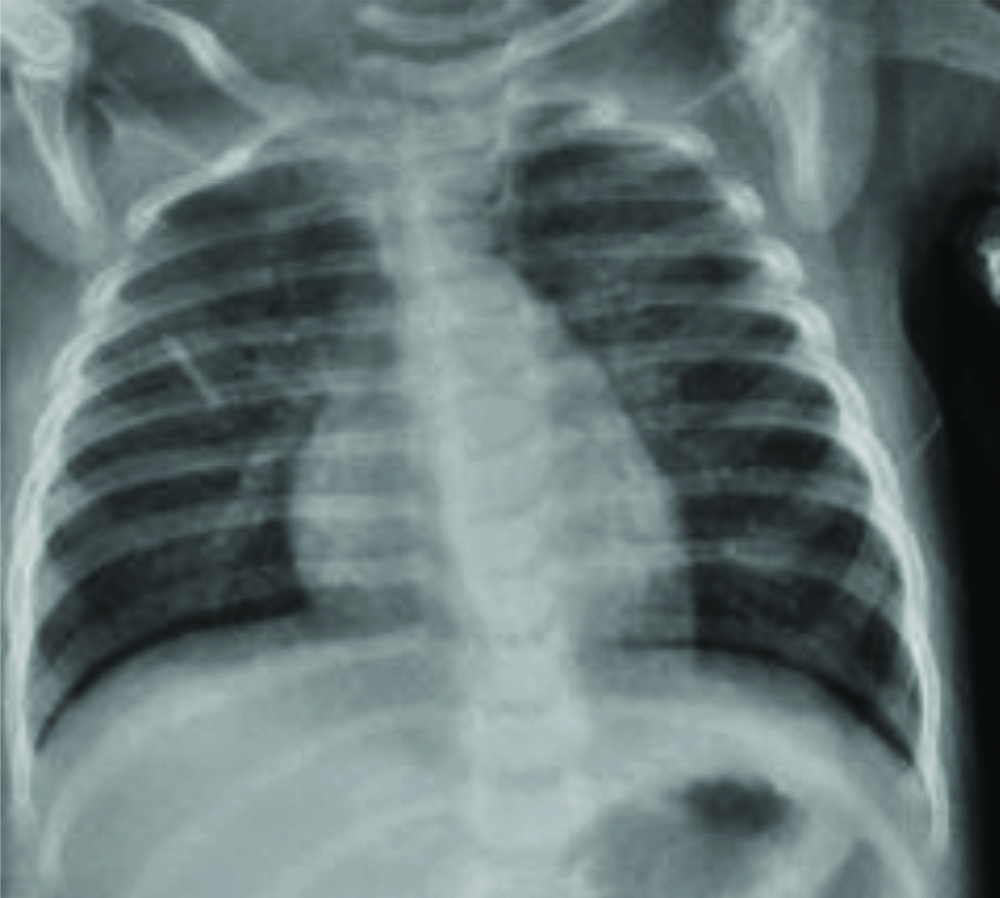
On examination, the child was fully alert but irritable with weight 5.6 kg, length 58 cm and was having cough with grunting. During auscultation of chest, bronchial breathing with decreased breath sounds was observed. There was presence of crepitation over the middle and lower lobes of both lung fields. The abdomen was soft with liver margin palpable 2 cm below the right costal margin. The neurological examination was otherwise unremarkable. Pulse oxymeter showed SpO2 of 84% while breathing room air. A presumptive diagnosis of acute bronchopneumonia with severe respiratory distress was made based on acute onset dyspnoea, decreased breath sound and chest x-ray [Table/Fig-1]. Parental consent was obtained.
The Chest X-ray of child at the time of admission showing bilateral patchy appearance with peri-bronchial thickening and poorly defined air-space opacities suggestive of bronchopneumonia.
On laboratory examination, white blood cell count was 10,000/mm3 with neutrophils 75% and marked lymphocytopenia. The serum electrolytes were well-maintained. C-Reactive Protein (CRP) levels were 4.8 mg/dL. The child was empirically started on parenteral antimicrobial chemotherapy. Inj ceftriaxone 250 mg i.v., twice daily and syrup azithromycin (200 mg/5 mL), 1.5 mL once orally, were administered along with Nebulisation with 3% normal saline every six hourly and 0.6 mL Salbutamol every 4-6 hourly for bronchodilation.
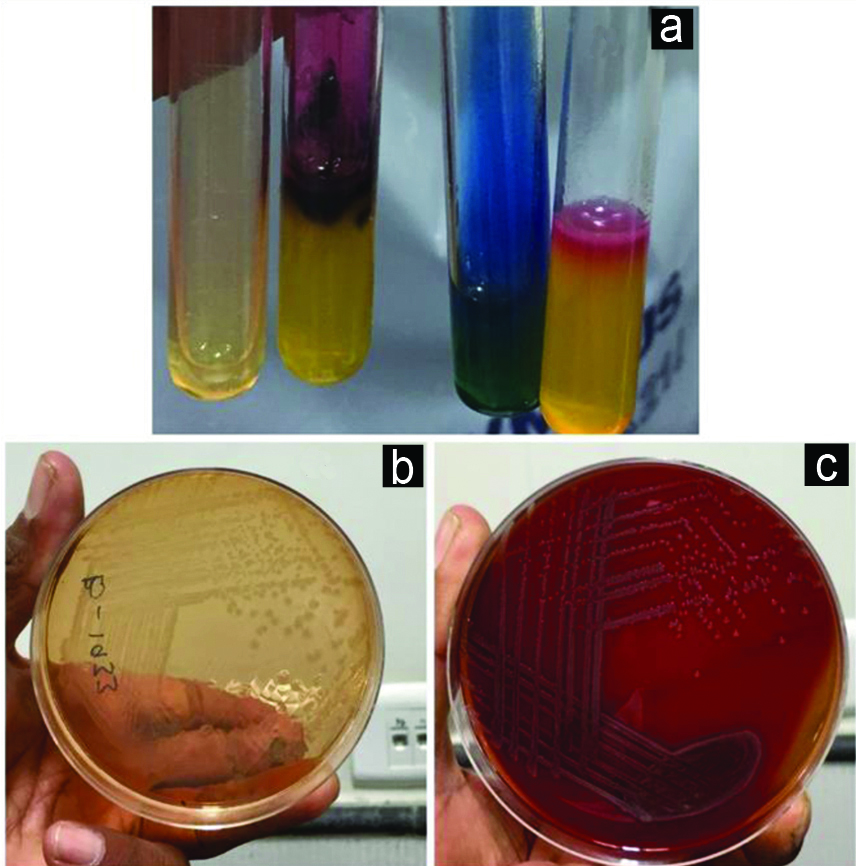
Blood culture sample was taken in BACTEC blood culture bottles on admission which flagged positive following 24 hours of incubation. The sample was processed according to standard Microbiological techniques on Blood agar and Mac-Conkey agar which yielded Gram-negative aerobic bacilli which was identified as Salmonella species using biochemical tests [Table/Fig-2] and VITEK 2 Gram Negative (GN) panel. On basis of phenotypic tests (i.e., citrate utilisation, gas production during glucose fermentation, adequate H2S in Triple Sugar Iron, both Lysine and ornithine decarboxylation) along with slide agglutination using polyvalent anti-sera; the isolate was reported as probable Salmonella enterica serovar Typhimurium. Any past history of diarrhoea was reconfirmed from the parents, but no history of loose stool was present. Stool culture was also done using Xyline Lysine Deoxycholate agar, MacConkey Agar and Selenite F broth, but no pathogen was isolated.
a) Biochemical reaction of the isolate: Urease: Negative, TSI: K/A with H2S, Citrate Utilisation Test: Positive, Mannitol Motility: Fermenter and Motile. b) Growth of isolate on MacConkey with Nonlactose fermenting colonies. c) Blood Agar Plate with nonhaemolytic grey moist convex circular colonies.
Antimicrobial susceptibility testing was performed by the Kirby Bauer disk diffusion method and Vitek 2 AST cards. The zone diameter breakpoints available in Clinical Laboratory Standards Institute Supplement M-100, 29th edition were used for interpretation of the results [1]. The isolates were sensitive to commonly used antibiotics, such a ceftriaxone (MIC <0.125 μg/mL), cefuroxime (MIC ≤4 μg/mL), and trimethoprim-sulfamethoxazole (MIC <20 μg/mL), cefepime (MIC ≤1 μg/mL), meropenem (MIC ≤0.25 μg/mL), chloramphenicol (MIC ≤8 μg/mL). Resistance was observed against ampicillin (MIC ≥32 μg/mL), ciprofloxacin (MIC ≥1 μg/mL) and nalidixic acid (MIC ≥1 μg/mL).
Initially, during the first two days after admission, the child developed worsening of respiratory function (severe dyspnoea, tachypnoea, and SpO2 at room air of 82%). The patient was treated with non-invasive Continuous Positive Airway Pressure (CPAP) ventilation. Third day suprasternal retractions and stridor were also observed. The child was continued on CPAP ventilation for the next two days. Nebulisation with adrenaline (0.1 mL/kg/dose in 1 in 10,000 solution of adrenaline mixed in normal saline to make a total volume of 3 mL) using oxygen flow of 8 L/min was done intermittently to relive bronchoconstriction [2].
Five days after his admission to the hospital the patient’s condition started improving and the child became afebrile with reduced cough and respiratory distress. Nebulisation with adrenaline was stopped and CPAP was withdrawn. C-Reactive Protein (CRP) also became negative. Syrup azithromycin was stopped on Day 5.
The clinical course of illness improved and the child was continued on injection ceftriaxone. The repeat blood culture sent after completion of 14 days of ceftriaxone therapy was sterile. The child was discharged after proper counselling of the parents.
Discussion
The NTS consist of the members of genus Salmonella which are not associated with typhoid fever. They are important food borne pathogens generally associated with self-limiting diarrhoea with low case fatality. Although in some cases especially those with risk factors i.e., malnourished infants, elderly, sickle cell disease, Human Immunodeficiency Virus (HIV) and recent attack of malaria; there could be bacteremia followed by focal infections in extra-intestinal sites such as meninges, joints, bones etc., [3-5]. The mode of transmission in most of these cases is consumption of contaminated food of animal origin (e.g., poultry, milk, egg etc.,). The most common serovars associated with invasive infection are S. Enteridis, S. Typhimurium and S. Dublin [6]. Here authors’ report a case of invasive NTS in an infant aged less than six months of age.
Invasive NTS (iNTS) diseases are increasing worldwide and have higher case fatality of 13.4% among under five years population [4]. The data on NTS invasive disease remain limited from developing countries of south-east Asia. One of the reasons for this low reporting could be failure of clinic-based surveillance to capture individual with NTS invasive disease and other could be non-availability of bacteriology culture based diagnostic services [4]. Isolation of NTS from extra-intestinal samples is still infrequently reported from India. Sudhaharan S, reported isolation of 27 NTS in extra-intestinal samples in the three years and S. Typhimurium was the most common isolate to be recovered during their study period [7]. There have been few reports regarding bronchopneumonia caused by NTS in the past [8,9]. In these most of these cases S. Typhimurium was found to be the most common NTS isolated in children and adults. In case of children, most of the cases were above six months of age. Infection under six months will be difficult in exclusively breast-fed children due to food borne nature of the illness [9]. In most cases of invasive NTS disease, organism gains entry through gastrointestinal tract and subsequently moves to extra-intestinal sites via blood with absence of any diarrhoeal illness during the course of disease [10]. In this case also, there was no history of diarrhoea. Even in patients with gastrointestinal illness almost 5% of individuals will develop bacteremia, or serious potential problem. The cautious clinical assessment for septic foci, additional blood cultures, and antimicrobial therapy for all children with bacteremia caused by NTS should be done to prevent any mortality [3]. The mode of transmission is ingestion of animal origin food, thus infection in below 6-month infants, who are exclusively breast-fed is very rare. In this case, ingestion of cow milk by the child could be one possible source that leads to infection in the child, and progressing to severe pneumonia in the infant. The gastrointestinal illness caused by these organisms is self-limiting which seldom requires antimicrobial chemotherapy, third generation cephalosporin viz., ceftriaxone is considered as the drug of choice in most of the cases of iNTS disease [3]. There, an increase in antibiotic resistance among NTS isolated in various clinical samples. In a study carried out in Southern India, it was observed that resistance to ceftriaxone was showing increasing trend among NTS over the last 10 years [11]. In a recently published report from Southern India, it was observed that S. Typhimurium was most prevalent serotype with 393 isolates (27.3%) recovered during the 19 years period with a consistent and stable distribution during the study period. It also showed that multidrug resistance has increased among NTS and there is major increase in last decade [12].
Antimicrobial resistance in NTS an important risk factor for hospitalisation and bacteraemia. In analysis of five years data from two surveillance systems in United States, Foodborne Diseases Active Surveillance Network (FoodNet) and National Antimicrobial Resistance Monitoring System (NARMS), it was observed that occurrence of hospital admission and bloodstream infection was more, after infection with drug resistant NTS. This finding was strongly associated in case of drug resistant Salmonella Typhimurium, which was the most common NTS serotype isolated in the study period [13]. The isolate recovered in present case was also resistant to ampicillin and fluoroquinolones. The rampant use of antimicrobial in agriculture and poultry farms is considered as one of the important drivers for upsurge of antimicrobial resistance [14].
Conclusion(s)
The NTS are emerging food borne infection worldwide. Invasive disease with unusual manifestations can lead to severe disease. It should be suspected in cases of febrile illnesses with history of consumption of contaminated food of animal origin (mainly eggs, meat, poultry, and milk). Parents need to be educated and counselled regarding the importance of boiling of milk, proper cooking of food and using safe water especially in case of top-up fed young infants to prevent this disease. The invasive NTS will not be necessarily preceded by diarrhoea. Thus, timely blood culture in case of febrile illness in top-up fed young infant is essential to correctly diagnose invasive NTS infection to prevent mortality.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes (Parental consent)
For any images presented appropriate consent has been obtained from the subjects. Yes (Parental consent)
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Dec 24, 2020
Manual Googling: Jan 10, 2021
iThenticate Software: Jan 20, 2021 (10%)
[1]. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests. CLSI supplement M100. 29th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2019 [Google Scholar]
[2]. Verma N, Lodha R, Kabra SK, Recent advances in management of bronchiolitisIndian Pediatr 2013 50(10):939-49.10.1007/s13312-013-0265-z24222284 [Google Scholar] [CrossRef] [PubMed]
[3]. Acheson D, Hohmann EL, Nontyphoidal salmonellosisClinical Infectious Diseases 2001 32(2):263-69.10.1086/31845711170916 [Google Scholar] [CrossRef] [PubMed]
[4]. Stanaway JD, Parisi A, Sarkar K, Blacker BF, Reiner RC, Hay SI, The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017The Lancet Infectious Diseases 2019 19(12):1312-24.10.1016/S1473-3099(19)30418-9 [Google Scholar] [CrossRef]
[5]. Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA, Global burden of invasive nontyphoidal salmonella disease, 2010Emerging Infectious Diseases 2015 21(6):941-49.10.3201/eid2106.14099925860298 [Google Scholar] [CrossRef] [PubMed]
[6]. Haselbeck AH, Panzner U, Im J, Baker S, Meyer CG, Marks F, Current perspectives on invasive nontyphoidal Salmonella diseaseCurrent Opinion in Infectious Diseases 2017 30(5):498-503.10.1097/QCO.000000000000039828731899 [Google Scholar] [CrossRef] [PubMed]
[7]. Sudhaharan S, Kanne P, Vemu L, Bhaskara A, Extraintestinal infections caused by nontyphoidal Salmonella from a tertiary care center in IndiaJournal of Laboratory Physicians 2018 10(04):401-05.10.4103/JLP.JLP_79_1830498311 [Google Scholar] [CrossRef] [PubMed]
[8]. Khan S, Kumar VA, Sidharthan N, Mehta A, Backer B, Dinesh KR, Salmonella Typhimurium pneumonia in a patient with multiple myelomaTropical Doctor 2015 45(2):135-36.10.1177/004947551456542725540162 [Google Scholar] [CrossRef] [PubMed]
[9]. Saeed NK, Salmonella pneumonia complicated with encysted empyema in an immunocompromised youth: Case report and literature reviewJ Infect Dev Ctries 2016 10(4):437-44.10.3855/jidc.706927131011 [Google Scholar] [CrossRef] [PubMed]
[10]. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA, Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in AfricaLancet 2012 379(9835):2489-99.10.1016/S0140-6736(11)61752-2 [Google Scholar] [CrossRef]
[11]. Pragasam AK, Anandan S, John J, Neeravi A, Narasimman V, Muthuirulandi Sethuvel DP, An emerging threat of ceftriaxone-resistant non-typhoidal salmonella in South India: Incidence and molecular profileIndian J Med Microbiol 2019 37(2):198-202.10.4103/ijmm.IJMM_19_30031745019 [Google Scholar] [CrossRef] [PubMed]
[12]. Jacob JJ, Solaimalai D, Muthuirulandi Sethuvel DP, Rachel T, Jeslin P, Anandan S, A nineteen-year report of serotype and antimicrobial susceptibility of enteric nontyphoidal Salmonella from humans in Southern India: Changing facades of taxonomy and resistance trendGut Pathog 2020 12:4910.1186/s13099-020-00388-z33110449 [Google Scholar] [CrossRef] [PubMed]
[13]. Varma JK, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalisationsJ Infect Dis 2005 191(4):554-61.10.1086/42726315655779 [Google Scholar] [CrossRef] [PubMed]
[14]. Taneja N, Sharma M, Antimicrobial resistance in the environment: The Indian scenarioIndian J Med Res 2019 149(2):119-28.10.4103/ijmr.IJMR_331_1831219076 [Google Scholar] [CrossRef] [PubMed]