The highest prevalence of oral cancer has been observed in Indian subcontinent with increased incidence in people younger than 40 years due to exposure to carcinogens such as betel nut extracts, smoking and chewing form of tobacco [1-3]. Oral cancer develops from OPMDs through rising grades of oral epithelial dysplasia to fatal invasive malignancy [4-6]. Unfortunately, 50% of the patients have regional or distant metastases at the time of diagnosis, which reflects a significant diagnostic delay [5].
Being rapid, simple, cheaper and easy, sediment cytology may provide early diagnostic value in such cases with the help of preparation of smears of sediments from the biopsy specimen fixatives, which have exfoliated cells from the biopsy cut surfaces. Sediment cytology is more suitably coined as biopsy sediment cytology because it is acquired from fixatives in which the biopsy specimens are received [7]. Examination of the sediment of fixatives and analysis of the cytological image with relevant clinical and radiographical data allow judgment in a couple of hours which is advantageous in case of OPMDs and Oral malignancies [8].
Sediment cytology has been effectively assessed in variety of lesions such as cervix and breast [9], oesophagus and stomach [10], bladder [11], lung [12], bone lesions [13], and ovarian neoplasms [8]. To the best of our knowledge, there are very less number of studies conducted on the sediment cytology in OPMDs and Oral Malignancies. Hence, the present study was conducted to estimate the efficiency of sediment cytology in biopsy of OPMDs and Oral Malignant lesions.
Materials and Methods
This is a prospective observational study conducted in the Department of Oral Pathology and Microbiology, K M Shah Dental College and Hospital, Sumandeep Vidyapeeth Deemed to be University from February 2015 to January 2016 after obtaining Ethical Approval from the institute (SVIEC/ON/Dent/SRP/15032). Total 30 oral lesions were reported and included in present study amongst which 15 were OPMDs and 15 were oral malignant lesions.
Inclusion criteria: 10% formalin fixed biopsies, punch biopsy, incisional biopsy and excisional biopsies, measuring more than 1×1 cm in size, received in cases with provisional diagnosis of oral pre-malignant lesions and oral malignant lesions during the time period of February 2015 to January 2016 were included in this study.
Exclusion criteria: Tissue received after more than 24 hours of biopsies were excluded from the study.
The material required for present study was left over formalin of specimen bottles in which the biopsy specimens were received. The obtained specimens were then shifted to a container with fresh fixative. The fixative fluid from the original specimen bottle was centrifuged at 3000 rpm for five min. The supernatant of the centrifuged material was discarded; and the sediments were washed two times in normal saline and used for smear preparation on albumen coated slides for good adherence of cells. According to the usual method for Cytology smear preparation, the prepared smears were first fixed in 95% ethanol with 3% glacial acetic acid (Biofix spray) and eventually were stained with Papanicolaou (PAP) stain [14].
According to Shafer’s, the cytological smears are classified under Class I-normal (only normal cells are observed), class II-atypia (presence of minor atypia due to inflammation with no signs of malignancy), class III-intermediate (wider atypia suggestive of severe dysplasia, carcinoma in-situ or cancer), class IV-suggestive of cancer (shows few epithelial cells with malignant changes in which biopsy is mandatory), Class V-positive for cancer (cells show characteristic malignant changes in which biopsy is mandatory) [15]. Based on this classification, the reporting of cytological smears was done as negative (Class I) or positive (Class II and III) or for OPMDs and as positive (Class IV and V) for oral malignant lesions. The prepared smears were analysed by two observers to reduce the inter-observer bias. The pre-malignant and malignant lesions were labelled according to the grade of dysplasia, for the features like increased cellularity, increased nucleo-cytoplasmic ratio, nuclear and cellular pleomorphism and hyperchromatic nuclei [Table/Fig-1,2 and 3] and were labeled as: Class I cytology, class II cytologic atypia and class III cytologic atypia for OPMDs and Class IV or V for oral malignant lesions. The cytological results were co-related with histopathological diagnosis taking the latter as bench mark [Table/Fig-1,2].
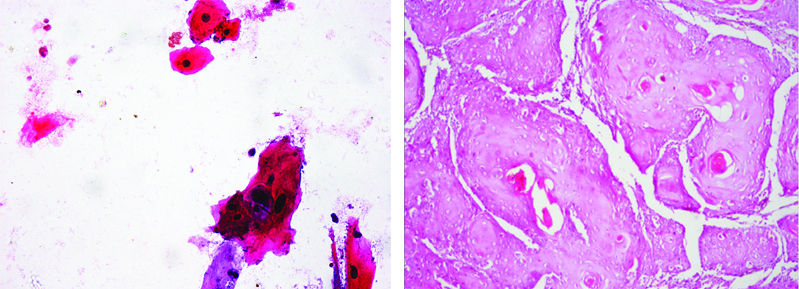
Cytology of OSCC Case Showing Dysplastic epithelial cells. (X100) and H&E stained (X40) histopathology section of the same case shows Malignant epithelial cells.
OSCC: Oral squamous cell carcinoma
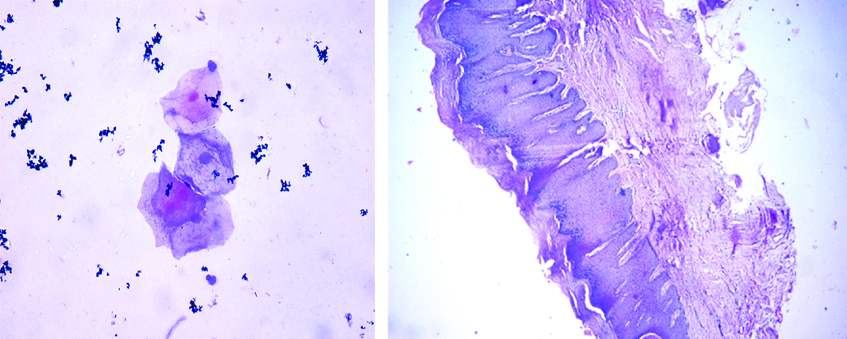
Cytology of oral leukoplakia case showing atypical epithelial cells (X40) and H&E Stained (X10) histopathology section of the same case shows moderate epithelial dysplasia.
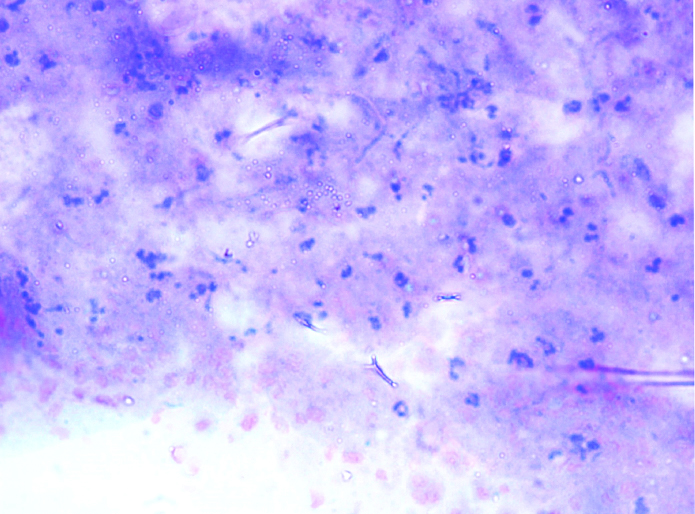
Showing inconclusive picture (X40).
Statistical Analysis
The data was entered into Microsoft Excel and descriptive statistics were used to analyse the data.
Results
A total of 30 cases were evaluated for the sediment cytology of OPMDs and oral malignant lesions. The documented clinical and histopathological data showed that 15 cases were diagnosed as malignant lesions all of which were oral squamous cell carcinoma and 15 were documented as the OPMDs out of which five cases of oral leukoplakia, one case of erythroplakia, seven cases of Oral Submucous Fibrosis (OSMF) and two cases of oral lichen planus were reported [Table/Fig-4].
Distribution of lesions according to gender.
| Lesions | Male | Female |
|---|
| Oral squamous cell carcinoma | 7 | 8 |
| Leukoplakia | 2 | 3 |
| Oral submucous fibrosis | 6 | 1 |
| Erythroplakia | 0 | 1 |
| Oral lichen planus | 1 | 1 |
The patients of study group were between 29 to 70 years of age with mean age of 54±17.38 years. Mean age of males and females were 53.77±15.43 years and 54.23±19.77 years, respectively [Table/Fig-4].
Out of 30 cases, 12 cases were positive, two cases were negative and one case was inconclusive to give any diagnosis for oral malignant lesions and only three cases were positive, nine cases were negative and three were inconclusive to give any diagnosis for OPMDs [Table/Fig-5].
Results of sediment cytology.
| Analysis by sediment cytology | Total | Positive | Negative | Inconclusive |
|---|
| Oral malignant lesions | 15 | 12 | 2 | 1 |
| Oral potentially malignant disorders | 15 | 03 | 9 | 3 |
| Total | 30 | 15 | 11 | 4 |
Fifteen cases showed true positive results, out of which 12 cases were malignant lesions and three were OPMDs. Markedly, nine false negative results were obtained; all of them are for OPMDs [Table/Fig-6].
| Values | True positive | True negative | False positive | False negative |
|---|
| No. of samples | 15 | 4 | 2 | 9 |
Discussion
The present study results revealed that, out of 15 malignant lesions, 12 lesions were properly diagnosed with 1 inconclusive result and only 3 out of 15 OPMDs were properly diagnosed with sediment cytology whereas three cases were inconclusive for diagnosis. Thus, sediment cytology was revealed to be precise in diagnosing 15 out of 30 lesions and an overall diagnostic accurateness of 50% was noticed.
Studies to evaluate the role of sediment cytology in bone lesions, conducted by Nagalotimath SJ et al., and Valiathan M et al., revealed diagnostic accuracy of 100% and 79%, respectively [9,16]. Shahid M et al., revealed a diagnostic accuracy of 90.3% in ovarian neoplasms [8]. Study conducted by Chaudhari VV et al., revealed that sediment cytology showed overall diagnostic accuracy of 85% in cases of oral benign and oral malignant lesions [7]. Hence, the present study results were almost nearby with above stated studies, showing an overall diagnostic accuracy of 50% and in case of oral malignant lesions the diagnostic accuracy was 80%, which is satisfactory for malignant lesions atleast. Due to the accuracy of only 20% in case of OPMDs, it is not suggestive to use sediment cytology as a diagnostic tool for OPMDs.
There are more than 7,80,000 cases of cancer around the globe while head and neck squamous cell carcinoma stands 5th in the cancer incidence list [6,17]. In developing Asian countries and under developed countries of Africa, maximum numbers of cases have been overlooked due to the deficiency of health services. Inspite of the intense and multidisciplinary treatment methods, along with preoperative or postoperative chemotherapy and radiotherapy with reconstructive surgery, there are very few cases seen with the five-year survival rate and maximum number of cases showed recurrence due to lymph node metastasis [18]. There is a long way to go to avoid the treatment failures like, distant metastases, second primary tumours and loco-regional recurrence [13,19,20]. Currently, the treatment approach is based on clinical, radiological, and histo-pathological assessment to determine the phase or grade of the disease. It is established that the lymph node metastasis is the most prominent and independent prognostic factor in head and neck squamous cell carcinoma indicating poor prognosis [21].
The most common OPMDs are oral leukoplakia, OSMF, oral lichen planus and oral erythroplakia, which we have also noticed in present study [6]. An 18% of OPMDs will develop into oral cancer; it has been shown that certain clinical sub-types of leukoplakia are at a higher risk for malignant transformation than others and varying malignant transformation rate from 0.13-17.5% [3]. In studies reported in recent years, the prevalence of oral leukoplakia varies between 1.1% and 11.7%, with a mean value of 2.9%. Proliferative verrucous leukoplakia, which is a form of verrucous leukoplakia, characterised by multi-focal presentation, has a strong potential for malignant transformation and is resistant to treatment [6]. Wide heterogeneity in requisites of clinical outcome and response to treatment still persists in patients who fit in to the same risk group.
Present study revealed a new technique sediment cytology for fast diagnosis of oral neoplasms. The technique has great importance in case of diagnosis of bone lesions, as it usually take long time for a routine histopathological report of such lesions because of the decalcification step in their histopathological laboratory processing. Within 1-3 hour of specimen receiving, one can provide a rapid preliminary diagnosis for such lesions [8]. It has also shown considerable results in studies for rapid diagnosis of ovarian neoplasms, where Fine Needle Aspiration Cytology (FNAC) is hardly ever performed [16]. Other than rapidity of diagnosis, the aided advantages of this technique is its simple procedure, which doesn’t require expensive equipment. Moreover, routinely preferred cytological PAP staining procedure is used in present study, which gives long lasting and adequate cellular structure details. With the help of such innovative technique, potentially valuable material is used rather than discarding. In addition, the technique can be preferred as an adjunct to frozen sections as, in a developing country like ours, where the availability of frozen section technique is rare, this easy, simple and cheap technique can be beneficial for rapid diagnosis.
Limitation(s)
Firstly, with the help of cytological smears the lesion can only be categorised as OPMD or malignant, but it cannot be used for definite diagnosis. Second limitation was, small sample size.
Conclusion(s)
The present study is the novel attempt to use the sediment cytology technique in OPMDs and oral malignant lesions. The results revealed that technique gives 80% diagnostic accuracy in case of oral malignant lesions which is quite an acceptable number but showed accuracy of only 20% in case of OPMDs. More studies should be conducted with larger sample size to appraise these techniques as screening method for the OPMD and Oral Malignancy.