Maintaining the bacterial stock culture is an essential part in microbiology laboratory for future processing, performing quality control and for research purposes [1,2]. Different bacterial species have varying ability to remain viable during storage ranging from few days to years. This is due to variable level of tolerance to change in environmental temperature, pH, osmolarity, oxidative stress and their own special metabolic need. Fastidious bacteria tend to become non-viable more easily than non-fastidious. There are different methods of bacterial stocking like cold storage, drying methods, freeze drying in vacuo (lyophilisation), etc., [3,4]. Cold storage under ultra-low freeze temperature (-20°C and -80°C) is the most common method used in clinical microbiology laboratories, because of the ease of performance and cost effectiveness. Cryo-preservation also known as cold storage in the presence of cryoprotectant can offer long term viability of bacterial and fungal cultures if repeated freeze thaw cycles are avoided [4-6].
Preferred temperature for long term storage of medically significant microbial strains would be an ultra-low freezer of -60°C or below [3,5]. There has been a documented evidence of genetic drifts and modification in strains stored above -60°C. Anti-microbial resistant Quality Control (QC) strains especially those with plasmid-mediated resistance, have been shown to lose the plasmid when stored long term at this condition [7]. According to International Organisation of Standardisations (ISO 11133) QC stocks and other strains should be stored in multiple portions at temperatures below -70°C or lyophilised. Strains stored at higher temperatures, has a reduced duration of viability and possibility of undergoing genetic drift or genetic modification. Repeated freeze-thaw cycles may compromise the genetic integrity of the organisms [8,9].
Organisms should be suspended in a liquid basal medium along with cryoprotective agents. SKM, BB, Brain Heart Infusion Broth (BHIB), Trypticase Soy Broth (TSB), Nutrient Broth (NB) are some of the media being used widely for stock preparation. Glycerol, Dimethyl Sulfoxide (DMSO), SKM and polyethylene glycol are some of the cryoprotective agents studied extensively [1,3,10-12]. PGB preparation was extensively studied for its enhancement of pigment production of certain bacteria. Overnight peptone water culture of clinical isolates is performed routinely in many laboratories to offer further biochemical testing of isolates. Hence, log phase culture of organisms will be available for storage, if peptone water is used as the base media. This avoids the time and resource required for preparing an exclusive storage medium.
Addition of polypropylene straws or cryobeads to the stocking fluid offers a surface for adsorption of the bacterial and fungal strains. Individual beads can be drawn out during every subculture avoiding repeated freeze thaw of the original stock as well as avoiding the contamination [13-16].
The current study compares the performance efficacy of cryobead storage method with three different basal media-peptone broth with 15% glycerol, BB with 15% glycerol and 10% SKM, used for suspending the organism. The longevity of the stored isolate in all three media, preservation of phenotypic characters and antibiotic susceptibility pattern in three different storage media were analysed.
Materials and Methods
This was a prospective analytical study, undertaken from December 2019 to October 2020, in the Department of Microbiology, SRM Medical College Hospital and Research Centre, Kattankulathur, Tamil Nadu, India with the Ethical Committee approval (IEC number:1840/IEC/2019).
Convenient sampling method was followed. Ten different isolates were used for studying the stocking methods. The isolates chosen were representative of different families of commonly isolated bacteria and one common yeast isolated in clinical labs. Both drug susceptible control strains and drug resistant clinical strains were studied. Three cryovial preparations were made for each stocking media (PGB, BGB and SKM) against each isolate. There were three vials per isolate per preparation totalling to 30 vials per preparation.
The following strains were included:
Escherichia coli ATCC 25922
Staphylococcus aureus ATCC 25923
Enterococcus faecalis ATCC 29212
Candida albicans
Klebsiella pneumoniae- Confirmed ESBL producer
Escherichia coli- AmpC producer
Klebsiella pneumoniae - Confirmed Carbapenemase producer (by modified carbapenem inactivation method)
Methicillin Resistant Staphylococcus aureus (MRSA)
Multidrug Resistant (MDR) Pseudomonas aeruginosa
Vancomycin Resistant Enterococcus faecalis (VRE)
The phenotypic characters of isolates were studied before storage and at stipulated time intervals during the storage period.
The bacterial cultures were studied for their phenotypic characters by gram stain [Table/Fig-1], colony morphology and other specific properties like haemolysis in Blood Agar (BA) and differential colour production in Mac-conkey Agar (MAC) and HiChrom UTI agar (UCA). Specific biochemical reactions were used for speciation of the isolates like catalase, oxidase test, indole and urease production, citrate utilisation, fermentation of mannitol, arabinose, sorbitol, reactions in Triple Sugar Iron agar (TSI), Bile esculin growth, nitrate reduction, Methyl red and Voges proskauer test, Coagulase test-tube and slide, salt tolerance 6.5% were also studied [1,17].
Gram stain image of Staphylococcus aureus (Magnification 1000x).
Candida albicans was studied by gram stain, germ tube test, colony morphology in Sabouraud Dextrose Agar (SDA), apple green colour in Candida Chrome Agar (CCA), chlamydospores production in Corn Meal Agar (CMA) [1,18].
Extended spectrum Beta lactamase, Amp C beta latamase and carbapenemase production was tested by combination disk method (ceftazidime/ceftazidime clavulanic acid difference ≥5 mm), cefoxitin screening (zone diameter ≤18 mm with D zone in ceftriaxone) and carbaNP test respectively. Methicillin resistance in Staphylococcus aureus was detected through cefoxitin (zone diameter ≤21 mm) disk screening. Vancomycin resistance in Enterococcus spp. was detected through vancomycin (zone diameter ≤14 mm) disk diffusion method [2]. Tests to detect special resistant patterns in other isolates were recorded as positive or negative.
Antimicrobial Susceptibility Testing (AST) was performed by Kirby Bauer disk diffusion method for the drugs listed under [Table/Fig-2] as per Clinical and Laboratory Standards Institute (CLSI) guidelines for bacterial isolates and Candida albicans [2,19].
List of drug tested for each organism.
| Organism | Antimicrobial test |
|---|
| Staphylococcus aureus ATCC 25923 | Benzyl penicillin, ampicillin, cefoxitin, ciprofloxacin, gentamicin, trimethoprim/sulphamethoxazole, tetracycline, erythromycin, clindamycin, vancomycin, teicoplanin, linezolid, bacitracin |
| Enterococcus faecalis ATCC 29212 | High dose gentamicin (HLG) |
| Escherichia coli ATCC 25922 | Ampicillin, cefoxitin, cefazolin, cefuroxime, ceftriaxone, cefotaxime, cefepime ceftazidime, amoxicillin clavulanic acid, piperacillin tazobactam, ertapenem, imipenem, meropenem, ciprofloxacin, ofloxacin, gentamicin, amikacin, trimethoprim/sulphamethoxazole, tetracycline |
| MRSA | Benzyl penicillin, ampicillin, cefoxitin |
| VRE | Vancomycin, teicoplanin |
| Klebsiella pneumoniae- ESBL | Ceftazidime/ceftazidime clavulanic acid, cefoxitin, ceftriaxone, cefotaxime, cefepime, amoxicillin clavulanic acid, piperacillin tazobactam |
| Escherichia coli- AmpC | Cefoxitin, ceftriaxone, cefotaxime, cefepime, amoxicillin clavulanic acid, piperacillin tazobactam |
| Klebsiella pneumoniae- Carbapenemase | Ertapenem, imipenem, meropenem, carbaNP test |
| MDR Pseudomonas aeruginosa | Ceftazidime, cefepime, piperacillin tazobactam, ciprofloxacin, gentamicin, amikacin, imipenem, meropenem, carbaNP test |
| Candida albicans | Fluconazole, itraconazole, voriconazole, amphotericin B |
ATCC: American type culture collection; VRE: Vancomycin resistant Enterococcus spp; MRSA: Methicillin resistant Staphylococcus aureus; ESBL: Extended spectrum betalactamase producer; MDR: Multidrug resistance
Results within the specified quality control ranges for American Type Culture Collection (ATCC) isolates were considered concordant and out of range were considered discordant. Interpretation of results was recorded as susceptible/Intermediate/Resistant for non ATCC isolates, tested with individual drugs. Reproducibility of the morphological, biochemical and AST results before and after storage was assessed by same methods during each revival. Percentage of concordance was analysed for each preparation.
Storage media preparation and sterilisation was done as per manufacturer’s instruction. The present study has adapted the concentration of individual components, combination of different bases and usage of glass beads from various studies in the past and currently available commercial preparations [10,11,13-15].
1. 10% Skim milk-stock preparation
Three mL cryovials containing 12 cryobeads, was sterilised by autoclaving at 121°C for 15 minutes. 10% skim milk (HiMedia) prepared by autoclaving at 121°C for 5 minutes. Heavy suspension of the individual isolates in 1 mL of skim milk was prepared using sterile cotton swab. The suspension was transferred to cryovials with beads.
2. Peptone glycerol (15%) broth-stock preparation
Three mL cryovials containing 12 cryobeads with 150 μL of glycerol added to 850 μL peptone water 1% (HiMedia) prepared by autoclaving at 121°C for 15 minutes. Heavy suspension of fresh overnight culture of individual isolates was emulsified using sterile cotton swab.
3. Brucella glycerol (15%) broth-stock preparation
Three mL cryovials containing 12 cryobeads and 150 μL of glycerol added to 850 μL BB (HiMedia) prepared by autoclaving at 121°C for 15 minutes [Table/Fig-3]. Heavy suspension of fresh overnight culture of individual isolates was emulsified using sterile cotton swab.
Image of cryobead vials of PGB preparations. (Obverse and reverse image of the cryobead vials).

All stock preparations were allowed to stay undisturbed at ambient temperature for 30 minutes allowing time for the isolates to settle in cryobeads. Later excess solution was pipetted out and vials were stored at -80°C throughout the study period.
Revival of stock: Revival of the stock was done in three different duration patterns to study the longevity of the stock with monthly, quarterly and once at end of 10th month, freeze thaw. Each set of preparation (PGB, BGB and SKM) was subjected to one pattern of revival. First set of stock was subjected to revival once in a month using one cryobead. The second set of stock was revived at once in three months frequency retrieving one cryobead each time. The third set was stored undisturbed and revived at the end of 10 months.
Cryovials were brought out of freezer and opened very briefly to remove one cryobead at a time and replaced back immediately. This avoided exposure of other cryobeads in the vial to complete thaw. The cryobead was inoculated in a 1 mL peptone broth and incubated at 37°C for 3 hours and then plated on to nutrient agar for isolation.
Longevity of the stock was assessed by the quality of revival (presence or absence of growth) and observable difference in growth. Phenotypic characters was assessed by biochemical reactions and antibiogram pattern generation for each revived strains as per standard guidelines [2,19].
Statistical Analysis
Statistical analysis was done by Fisher-Exact test using Statistical Package for the Social Sciences (SPSS) software version 27.0.
RESULTS
Over a period of 10 months, 10 isolates were stocked in triplicates of each stocking preparations studied, accounting for 30 stocks per method. Revived isolates were assessed for confluence in growth and time taken to revive. [Table/Fig-4] shows the percentage of revival (confluent growth at 24 hours) of different isolates in all three preparations tested at three different frequency of interval. There was no contamination of the isolates observed in all stocked cryobead preparations. All isolates stored in PGB and BGB were able to revive at 24 hours of incubation with confluent growth irrespective of the frequency of revival. Monthly and quarterly revival, leading to a transient freeze-thaw didn’t affect the viability of the stock.
Percentage of revival of individual isolates stored in all three preparations under monthly, quarterly and after 10 months patterns.
| A | B | C | D | E | F | G | H | I | J | Revival with confluent growth (%) |
|---|
| Revival pattern | Preparation | | | | | | | | | | | N=100 |
| Monthly (n=10) | PGB | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 100 (100%) |
| BGB | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 100 (100%) |
| SKM | 10 | 8* | 10 | 10 | 10 | 10 | 10 | 8* | 8* | 10 | 94 (94%) |
| Quarterly (n=3) | | | | | | | | | | | | N=30 |
| PGB | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 30 (100%) |
| BGB | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 30 (100%) |
| SKM | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2* | 3 | 29 (96.67%) |
| 10th month (n=1) | | | | | | | | | | | | N=10 |
| PGB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 (100%) |
| BGB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 (100%) |
| SKM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0* | 1 | 9 (90%) |
A-Staphylococcus aureus ATCC 25923, B-Enterococcus faecalis ATCC 29212, C-Escherichia coli ATCC 25922, D-Methicillin resistant Staphylococcus aureus, E-Vancomycin resistant Enterococcus faecalis, F-Escherichia coli (Amp C), G-Klebsiella pneumoniae (ESBL), H-Klebsiella pneumoniae (carbapenemase), I-Pseudomonas aeruginosa (MDR), J-Candida albicans
n-Number of times revival performed under this pattern for individual isolates
N-Number of times revival performed under this pattern for individual preparation
*Inability to revive with confluent growth and requiring enrichment for subsequent 24 hours to produce confluent growth
In SKM, there was a slight observable decrease in quantity with monthly revival after 9th month with three isolates (Pseudomonas aeruginosa, Klebsiella pneumoniae and Enterococcus faecalis) and one isolate (Pseudomonas aeruginosa) at quarterly and 10th month revival at first 24 hours. Overnight culture of cryobeads in peptone water had produced moderate growth after 48 hours of incubation. All other bacterial and Candida isolate had no observable difference. The isolates stored in PGB compared to the SKM demonstrated a significantly better revival rate, p=0.03. The result was statistically significant (p<0.05). Performance of PGB and BGB was equally efficient (p=1).
On observing revival of individual isolates, SKM preparation had 83.33% overall revival rate in terms of confluence in growth at the end of 24 hours. Three out of ten isolates failed to produce growth towards the end of monthly revival. Similarly, one out of ten isolates failed to produce growth with both quarterly revival and single revival at 10th month.
Phenotypic character of the isolates assessed through the key biochemical reactions used to speciate the isolates and antimicrobial resistance properties, remained stable with all 10 isolates in all three revival patterns in all three preparations. Results were similar to the pre-storage condition without alteration. The total number of different morphological, biochemical and antimicrobial susceptibility/resistance properties was tested for individual organisms and their percentage concordance between the results before and after storage for each preparation. Each isolate stored in preparation was tested in 14 (10+3+1) episodes of revival. All these properties were retained to a 100% in all three preparations.
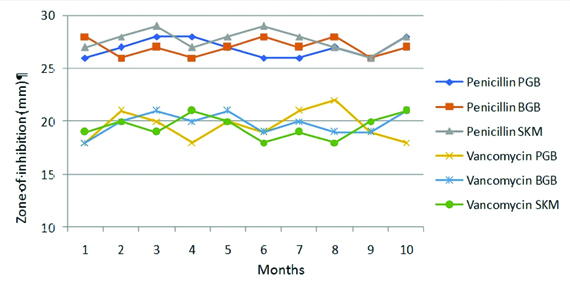
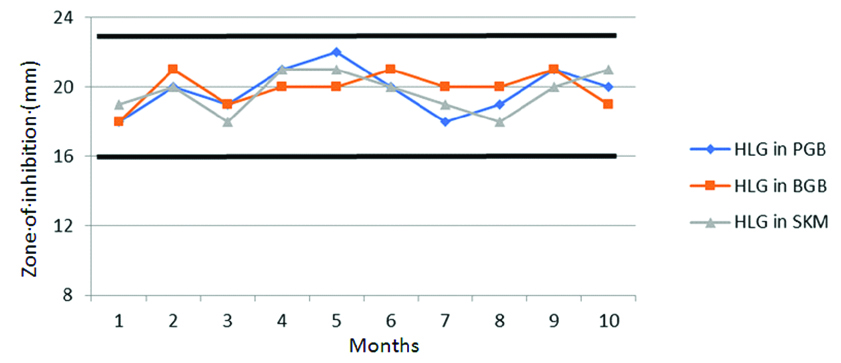
Pattern of AST of the isolates were studied with each revival. Thirty nine different drugs were studied for zone diameter variations using ATCC strains and one Candida albicans isolate. The strains stocked in all three preparations produced values within the acceptable range. Escherichia coli ATCC 25922 were tested for 20 different antibiotics, Staphylococcus aureus ATCC 25923 for 14 drugs, Enterococcus faecalis ATCC 29212 for High level gentamicin drugs and Candida albicans for four drugs. The results were well within the expected range and there was no discernible drift on mapping the zone of inhibition observed with all three preparations. [Table/Fig-5,6] shows the mapped zone of inhibition of Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 29212 stocked in all three preparation for penicillin, vancomycin and HLG with monthly testing. All results were found to be within the desired QC range and without unidirectional drift.
Mapping the zone of inhibition of Staphylococcus aureus ATCC 25923 stocked in PGB, BGB and SKM for the drugs Penicillin and Vancomycin.
Quality control range for Penicillin: 26-37 mm and Vancomycin: 17-21 mm
Mapping the zone of inhibition of Enterococcus faecalis ATCC 29212 for the drug gentamicin (120 μg) for High level Aminoglycoside resistance detection.
Quality control range for HLG: 16-23 mm
Special resistance patterns of Methicillin resistance in Staphylococcus aureus, ESBL and CRE in Klebsiella pneumoniae, AmpC beta lactamase in Escherichia coli, vancomycin resistance in Enterococcus faecalis were well preserved in PGB, BGB and SKM. Freeze thaw of different patterns had no significant effect on this resistance pattern of the isolates.
Discussion
Maintaining the viability and phenotypic properties- metabolic and AST of bacterial and yeast strains is crucial for carrying out researches. Cryobead method of preservation is the most common method followed for long term preservation of cultures in most laboratories. Beads decreased the possibility of contamination and also the repeated complete freeze thaw cycles. Results from past study shows that usage of cryobeads or glass beads had increased the recovery rate of isolates to 100% with non-fastidious and 80-100% in fastidious bacteria [13-15]. Fungal isolates have also shown similar results with cryobead technique [20]. Similar results were observed in the current study. Pipetting out the excess broth left the bead non-sticky. In the current study it was observed that, there was ease in retrieval of single bead minimising the freeze thaw duration of rest of the contents in the vial. This might be one of the reasons for best performance of PGB and BGB.
Glycerol and SKM are well known cryoprotective agents [21]. In this study, Glycerol based combination is found to offer better cryoprotective effect than SKM. Isolates stocked in both PGB and BGB preparations were having 100% viability in monthly, quarterly and long term (10th month) revival. Similar results were observed with other studies testing fastidious bacteria stock cultures. Most of the studies have analysed the various glycerol based preparations and found to produce 100% revival at -80°C [9,10,22]. These studies have experimented among different concentrations of glycerol with or without base media combinations and their findings are consistent with the current study. However, none of these studies compared glycerol with SKM.
A study comparing glycerol and SKM by Cody WL et al., had concluded that SKM was better than glycerol. However, the study was analysing strains left at room temperature for months dues to freezer failure. SKM preparations were found to perform better than glycerol in terms of viable colony count while tested for a shorter duration of 49 days by Cody WL et al., [11]. The discrepancy observed during long term analysis in the current study serves as a potential area of interest for further studies in future.
Base media containing micronutrients are needed to keep the isolates viable for long term. In the current study peptone base was found to be as effective as the Brucella base. Both peptone base and Brucella base preparations had a 100% revival rate within 24 hours. SKM was observed to produce overall revival rate of 83.33% on isolate-based analysis and 93.56% on frequency of revival based analysis. Of the 30 stock isolates stored in SKM preparation 25 revived at the end of 24 hours. There was slightly lower efficacy observed with the SKM preparation in terms of time required to revive and produce a confluent growth. Pseudomonas aeruginosa isolate had a decreased revival count in monthly, quarterly and at the end of 10th month with SKM.
The phenotypic properties of the 10 strains were kept intact throughout the study period by all three preparation. The drug resistance is the most vital properties in a clinical laboratory. Three ATCC strains tested against the panel of common drugs retained their QC ranges. Isolates with special resistance patterns produced consistent results during each revival. Average variation in zone diameter of the individual drugs tested against isolates was ±3 mm. There was no discrepancy in the sensitive/resistant results observed after each revival.
In all stocking methods some portion of bacterial and fungal isolates undergo physiological dormancy in due time and some portion becomes killed in the process of freezing. Ideal stocking method should be able to minimise the proportion of dying strains, during the preservation process as well as have a significant recovery rate in long term [16]. All three preparations used in the current study had good recovery rates. However, PGB and BGB proved better than the SKM which is one of the commonly used stocking media. PGB preparation was able to preserve the phenotypic properties as effectively as other two preparations.
The cost of preparation of BGB was highest followed by PGB. SKM was the cheapest method. PGB preparation is cost effective compared to the equally well performing BGB preparation.
Limitation(s)
Presence or absence of confluent growth was recorded, as the end-observation, in the current study. Exact viable colony count analysis before and after storage was not performed.
Conclusion(s)
Current study showed that PGB preparation could be used as an alternative to BGB and SKM in cryobead stocking method for bacterial and yeast isolates. The better stock preparation medium that supported long term viability was peptone with 15% glycerol, Brucella with 15% glycerol and 10% SKM, where the former two had a higher recovery rate. This study also showed that glycerol based preparations had a better cryoprotective effect than SKM preparation. Peptone based stock preparation would be an easily available, cheap, less time-consuming procedure in diagnostic laboratories if this methodology is adopted. Further study with a larger sample size of individual bacterial species is required to expand the inference.
ATCC: American type culture collection; VRE: Vancomycin resistant Enterococcus spp; MRSA: Methicillin resistant Staphylococcus aureus; ESBL: Extended spectrum betalactamase producer; MDR: Multidrug resistance
A-Staphylococcus aureus ATCC 25923, B-Enterococcus faecalis ATCC 29212, C-Escherichia coli ATCC 25922, D-Methicillin resistant Staphylococcus aureus, E-Vancomycin resistant Enterococcus faecalis, F-Escherichia coli (Amp C), G-Klebsiella pneumoniae (ESBL), H-Klebsiella pneumoniae (carbapenemase), I-Pseudomonas aeruginosa (MDR), J-Candida albicans
n-Number of times revival performed under this pattern for individual isolates
N-Number of times revival performed under this pattern for individual preparation
*Inability to revive with confluent growth and requiring enrichment for subsequent 24 hours to produce confluent growth