Though less common than community acquired pneumonia, healthcare associated or nosocomial pneumonia is the second leading cause of hospital acquired infections. This subgroup of pneumonia is increasing with increasing use of assisted ventilation of critically-ill patients and increasing prolonged length of inpatient hospital stay [1]. HAP is defined as pneumonia that occurs 48 hours or more after hospital admission and not incubating at the admission time. VAP is defined as pneumonia that occurs more than 48 to 72 hours after tracheal intubation. It affects 10% to 20% patients receiving mechanical ventilation for more than 48 hours [1,2]. HCAP is a recently recognised entity which is associated with longer duration of stay in healthcare facilities, rehabilitation facilities and dialysis centres.
In low and middle-income countries like India prevalence of Hospital-Acquired Infections (HAIs) vary between 5.7% and 19.1%, with a pooled prevalence of 10.1% [3,4].
From a microbiological standpoint, GNB account for about 70% of HAP, VAP and HCAP. Pseudomonas aeruginosa, Acinetobacter baumannii, and members of the family Enterobacteriaceae are the most common gram-negative organisms associated with nosocomial pneumonias. In the recent years, the carbapenem class is an important broad spectrum antibiotic that used in treatment of the infections due to these organisms [1]. However, increased use of carbapenems has led to selection of organisms harbouring ESBLs and Amp C β-lactamases leading to CR [5]. Nosocomial pneumonias caused by these CR bacilli pose an important challenge to clinicians due to their restricted therapeutic options. However, relatively few studies have been conducted to assess the prevalence of CR GNB causing HAP, VAP and HCAP in a low and middle-income like India where the standards of healthcare are often heterogeneous. Regional data in infectious disease need not reflect global trends especially with respect to mortality.
It is with this background, that the study was done at a leading Tertiary Care Centre in Southern India to assess risk factors and clinical outcomes in inpatients admitted in the Intensive Care Unit (ICU) with HAP, VAP and HCAP secondary to Carbapenem Resistant (CR) Gram Negative Bacilli (GNB).
Materials and Methods
This was a prospective cohort study conducted in medical and surgical ICUs in a Tertiary Care Hospital in Southern India from February 2015 to September 2016. Based on the resistance rate in GNB from a study by Gupta E et al., and with 95% Confidence Interval (CI) and 20% allowable error, minimum sample size required was 65 [6]. Ethical clearance was obtained from the Institutional Ethical Committee (IRB-AIMS-2014-287) prior to study initiation.
Patients admitted in the ICU during this period with pneumonia were selected. Pneumonia was defined as any hospitalised or ventilated patient who had purulent sputum (or endotracheal secretions), new radiological infiltrates, a core temperature of more than 38.3°C, and a leucocytosis or leukopenia [7]. All patients who had any of these three clinical syndromes as defined by American Thoracic Society and the Infectious Diseases Society of America [8] were further selected:
HAP is defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission.
VAP refers to pneumonia that arises more than 48-72 hours after endotracheal intubation.
HCAP included any patient who was hospitalised in an acute care hospital for two or more days within 90 days of the infection; resided in a nursing home; or attended a hospital or haemodialysis clinic; received recent intravenous antibiotic therapy or chemotherapy within the past 30 days of the current infection.
In addition, all included patients who had positive microbiological confirmation for a GNB either in a sputum or BAL quantitative culture with bacterial count exceeding 1,00,000 Colony Forming Units (CFU) per millilitre in the specimen.
Patients with recurrence of infection during the hospital stay, presenting with feature of sepsis, with polymicrobial infections (isolation of GNB along with gram positive or fungal organism), with other obvious focus of infection (for example, Urinary Tract Infection) were excluded from the study. Patients tracheotomised prior to the development of the nosocomial pneumonia were also excluded.
Measurements and outcome measures: Organisms were isolated according to their biochemical characteristics (manually or using Vitek 2 automated system-bioMerieux, Marcy l’ Etoile, France). The details recorded included age, sex, previous hospital admission in past one month, previous antibiotic therapy lasting for more than 72 hours in past one month. In addition, details of any use of carbapenems (imipenem, meropenem, doripenem and ertapenem) in past month were collected. The various co-morbid illnesses like type 2 diabetes mellitus, systemic hypertension, dyslipidaemia, coronary artery disease, chronic liver disease and obstructive airway disease were recorded.
All patients were followed-up for following outcomes-28 days at mortality, development of sepsis (blood stream infection), need of inotrope support, incidence of tracheostomies, length of stay in hospital and duration of mechanical ventilation for the patients. Each patient was also followed-up for concordance between the initial empirical antibiotic prescribed and the antibiotic sensitivity noted in the subsequent culture report.
Statistical Analysis
Data was analysed using OpenStat 30.0. Categorical data was summarised using proportion and percentage. Continuous data was summarised using mean and Standard Deviation (SD). All clinical factors and outcomes were compared between CS and CR groups using Independent t-test, Mann-Whitney test, Chi-square test or Fischer-Exact test as applicable. Antibiotics concordance was compared for occurrence of mortality using Chi-square also. The p-value <0.05 was considered statistically significant.
Results
A total of 130 cases of pneumonia were encountered in the ICU during the study period, of which 66 cases were of HAP, VAP and HCAP satisfying the inclusion and exclusion criteria. The demographics, co-morbid condition and outcomes of the cohort are shown in [Table/Fig-1]. It can be noted that exposure to previous antibiotics was a significant risk factor for CR (p=0.017). It can also be noted that all outcome measures-mortality (28 days), occurrence of sepsis, the need for inotrope usage, length of stay and duration of mechanical ventilation were worse for patient with CR (p-value=0.026, 0.035, 0.002, 0.041 and 0.005, respectively).
Demographics, co-morbidities and outcomes stratified for carbapenem resistance.
| CR (n=36) | CS (n=30) | p-value |
|---|
| Demographics |
| Age in year (Mean±SD) | 61.75±14.3 | 56.10±17.01 | 0.147 |
| Sex |
| Male | 25 (37.8%) | 26 (39.4%) | 0.084 |
| Female | 11 (16.7%) | 4 (6.1%) |
| Co-morbid conditions |
| Type 2 diabetes mellitus | 16 (44.4%) | 14 (46.7%) | 0.857 |
| Hypertension | 16 (44.4%) | 11 (36.7%) | 0.522 |
| Dyslipidemia | 5 (13.9%) | 7 (23.3%) | 0.522 |
| Coronary artery disease | 10 (27.8%) | 8 (26.7%) | 0.920 |
| Chronic obstructive airway disease | 3 (8.3%) | 2 (6.7%) | 0.799 |
| Bronchial asthma | 3 (8.3%) | 3 (10%) | 0.815 |
| History of cancer | 3 (8.3%) | 5 (16.7%) | 0.302 |
| Chronic kidney disease | 2 (5.6%) | 5 (16.7%) | 0.144 |
| Chronic liver disease | 6 (16.7%) | 0 (0%) | 0.019 |
| Clinical risk factors |
| Prior hospitalisation | 31 (86.1%) | 22 (73.3%) | 0.194 |
| Prior antibiotic usage | 30 (83.3%) | 17 (56.7%) | 0.017 |
| Prior carbapenem usage | 12 (33%) | 6 (20%) | 0.226 |
| Clinical outcome measures |
| Mortality (28 days) | 50% | 23.3% | 0.026 |
| Occurence of sepsis | 36.1% | 13.3% | 0.035 |
| Need for inotrope usage | 61.1% | 23.3% | 0.002 |
| Length of stay in days (Mean±SD) | 20.08±9.50 | 15.63±7.46 | 0.041 |
| Duration of mechanical ventilation in days (Mean±SD) | 11.81±8.13 | 6.83±3.46 | 0.005 |
| Lack of concordance of initial antibiotic therapy | 32 (88.9%) | 16 (53.3%) | 0.001 |
T-test and Mann-Whitney test were used for comparison of age, length of stay and duration of mechanical ventila-tion while Chi-square test was used for all other parameters; p-value <0.05 was considered significant
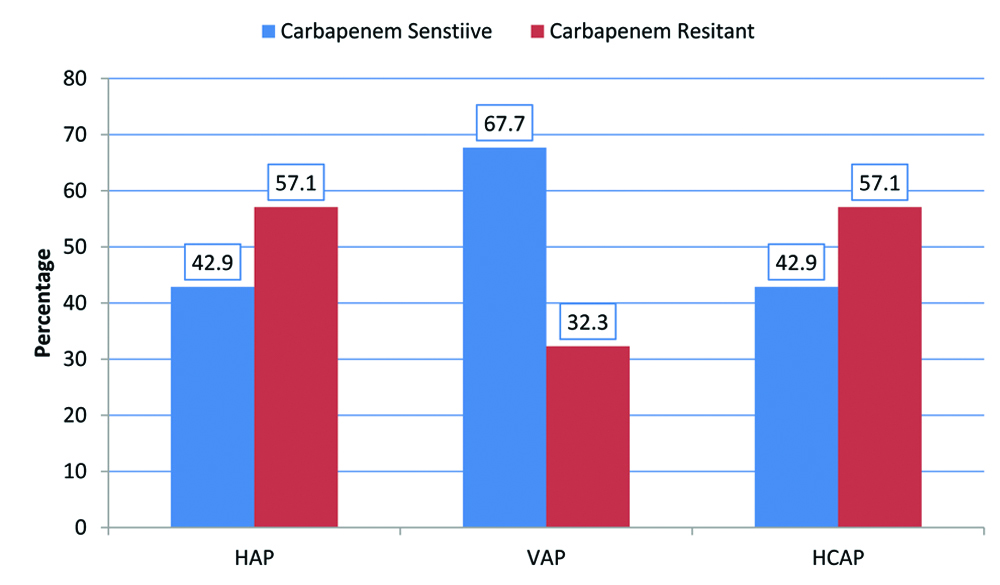
The most common GNB was found to be Klebsiella pneumoniae (n=30, 45.45%). This was followed by Acinetobacter baumannii (n=14, 21.2%), Pseudomonas aeruginosa (n=13, 19.7%) and Escherichia coli (n=5, 7.6%). Two cases of Burkholderia cepacia and 1 case of Enterobacter sp. and Stenotrophomonas maltophilia each were also encountered. Within this cohort, there were 36 cases (54.5%) GNB isolates with CR. It was most common in Acinetobacter baumannii (n=11 of 14, 78.5%), followed by Klebsiella pneumoniae (n=20 of 30, 66.6%), Escherichia coli (n=2 of 5, 40%) and Pseudomonas aeruginosa (n=3 of 13, 23.1%). [Table/Fig-2] shows occurrence CR with respect to the three syndromes (HAP, VAP and HCAP). CR was noted in all three syndromes- HAP (n=12 of 21, 57.1%), VAP (n=10 of 31, 32.3%) and HCAP at (n=8 of 14, 57.1%).
Prevalence of carbapenem resistance with respect to the type of pneumonia.
Discussion
This single centre prospective study showed that amongst all of the various clinical risk factors studied; it is the presence of co-morbid chronic liver disease (p=0.019) and prior usage of carbapenems within the past one month (p=0.017) which were associated with significant risk of development of CR. Data from key India studies published about CR in GNB organism are summarised in [Table/Fig-3] [6,9-16]. While there are many microbiology and genotypic based Indian studies, there are few studies addressing risk factor comparison for the development of CR in HAP VAP and HCAP which this study aims to address.
Key India studies on carbapenem resistance [6,9-16].
| S. No | Author | Study population (n) | Key outcome measure | Key findings on carbapenem resistance |
|---|
| 1. | Datta S et al., [9] | Blood culture postive neonatal sepsis secondary to Enterobacteriaceae (n=105) | Antibiotic susceptibility, genotypic detection | 14% of isolates showed CR NDM-1 genotype identified in all |
| 2. | Sinha N et al., [10] | All clinical isolates that grew Acinetobacter baumanni (n=140) | Antibiotic susceptibility, genotypic detection | 20% of isolates showed CR. IMP-1 and VIM-1 genotypes identified. |
| 3. | Sekar R et al., [11] | All clinical isolates that grew E. Coli and Klebsiella spp (n=2292) | Antibiotic susceptibility | 3% of isolated showed CR |
| 4. | Jaggi N et al., [12] | All clinical isolates that grew E. Coli and Klebsiella spp (n=528) | Antibiotic susceptibility, genotypic detection | 29.5% of isolates were CRNDM-1 and OXA-48 genotype identified in all |
| 5. | Veeraraghavan B et al., [13] | Culture postive sepsis 2° to CR K. pneumoniae spp (n=115) | Genotypic detection, mortality | 95% of CR infections were noscomial with 68% mortality.NDM-1 and OXA-48 genotype identified. |
| 6. | Gupta E et al., [6] | All clinical isolates of PseudomonasAcinetobacterE. coli and Klebsiella spp who were ESBL positive (n=2626) | Antibiotic susceptibility | Overall 22.16% of isolates showed CR.37.6% of Pseudomonas spp having the highest individual CR. |
| 7. | Gurjar M et al., [14] | VAP due to CR Acinetobacter sp (n=87) | Mortality | 46% mortality was noted |
| 8. | Devi P et al., [15] | ICU paitents with GNB respiratory infections | Incidence | 44% incidence noted,Acinetobater spp and Pseudomonas spp were the most common |
| 9. | Nabarro LEB et al., [16] | Paediatric CR Enterobacteriaceae sp bloodstream infection | MortalityRisk factor comparisonGenotypic detection | Mortality was 52%46% had a malignancy.NDM-1 genotype was most common |
These risk factors for CR were similar to those reported in literature especially the prior usage of carbapenems within the past one month. Richter SE et al., showed that receipt of any carbapenem in the prior 30 days was associated with the development of CR [17]. In another study by Ye J-J et al., showed a similar association between the development of Imipenem resistance in Acinetobacter baumannii and prior impipenem exposure [18]. Therefore, from a therapeutic standpoint, CR infections should be considered in the subset of pneumonia patients who have history of having received carbapenems within one month of their hospital admission.
It was also seen that patients with CR pneumonia were more likely to have received an ineffective initial antibiotic regimen than patients with CS pneumonia (p=0.001). This could explain the higher mortality which was noted in the CR pneumonia group; with almost 50% of the study patients succumbing to their illness. However, higher mortality with CR is reflected in other cohorts across the world and is summarised in a recent meta-analysis by Falagas ME et al., which studied mortality attributable to Carbapenem-Resistant Enterobacteriaceae. This study had reported mortality of 26%-44% in the studies which were included in the meta-analysis [19]. Pneumonias caused by these CR strains were found to have significantly higher incidence of other secondary outcomes (occurrence of sepsis, need for inotrope usage, length of inpatient stay and duration of mechanical ventilation). These are likely representative of the increased virulence of the CR organisms and might need combination therapy to avoid adverse outcomes [19]. Whether critically-ill patients with multiple risk factors for development of multiple drug resistance should be initiated upfront with a carbapenem-colistin combination for better outcomes needs to study in a well-designed study.
Limitation(s)
As a tertiary care centre, with a greater number of referral cases from different hospitals, microbiological flora and antibiotic sensitivity may show an institutional pattern which might not be applicable to population at large. Another limitation noted is the poor documentation of treatment received, exact nature and duration of antibiotics therapy received from previous hospitals could not be elicited in all cases.
Conclusion(s)
This study showed the high prevalence of CR amongst GNB isolates causing hospital acquired, ventilator acquired and HCAP. Exposure to previous antibiotics was a risk factor for development of CR. CR had led to significantly higher mortality, increased economic burden due to prolonged stay in hospital and higher chance of acquiring hospital acquired infections due to increased mechanical ventilator duration. Antibiotic policies should be revised and strictly implemented so that indiscriminate use of carbapenems, anti-pseudomonals and fluroquinolones are curbed. Antibiotic stewardship is the need of the hour.
T-test and Mann-Whitney test were used for comparison of age, length of stay and duration of mechanical ventila-tion while Chi-square test was used for all other parameters; p-value <0.05 was considered significant