Ideally, general anaesthesia should provide quick and pleasant induction, stable operating conditions, minimal adverse effects, rapid and smooth recovery of protective reflexes and psychomotor functions. Till recently, inhalational agents have been the choice for maintenance of anaesthesia due to the availability of sophisticated delivery systems which allowed the anaesthesiologists to have a fine degree of control on the concentration of anaesthetic agent administered to the patient. With the invention of newer induction agents and opioids having shorter half-life, with advents of infusion pumps and depth of anaesthesia monitors like BIS, TIVA is gaining popularity day by day [1].
Various forms of TIVA have been tried since the introduction of barbiturates into anaesthetic practice in 1930s [2]. The properties like faster recovery and minimal postoperative complications made propofol a very popular intravenous anaesthetic agent. However, one of the known side-effects of propofol is to cause profound arterial hypotension [3]. So, the technique of co-induction using two or more agents to induce anaesthesia has been studied [4]. The potential benefits of co-induction would mean that anaesthesia could be induced with a smaller combined dose of anaesthetic agents with fewer side-effects. This combination aims to capitalise primarily on the hypnotic and analgesic properties.
Lack of analgesic properties of propofol has necessitated the need for supplementary analgesic agents during TIVA. Various combinations of drugs have been tried which include midazolam-ketamine, propofol-ketamine, propofol-fentanyl and many more each with varying results. Fentanyl is used extensively in TIVA now-a-days. It belongs to opioid group of drugs and as a part of balanced anaesthesia it relieves pain, reduces somatic and autonomic response to airway manipulation [5]. Ketamine, a phencyclidine derivative is found to be a powerful analgesic even in subanaesthetic doses [6]. It also potentiates opioid analgesia via N-Methyl-D-Aspartate (NMDA) receptor blockade. Propofol-ketamine combination in TIVA has been tried but excitatory side effects were observed when ketamine was given at a dose of 1 mg/kg [7].
The present study was done to compare the effects of fentanyl or subanaesthetic dose of ketamine when given along with propofol on intraoperative anaesthetic requirement (primary outcome), postoperative analgesia and recovery profile (secondary outcome).
Materials and Methods
A randomised, double blinded study was conducted in patients who underwent elective laparotomy lasting less than four hours duration under TIVA. The study was conducted in Surgical Gastroenterology operation theatre of Sri Venkateswara Institute of Medical Sciences (SVIMS) university teaching hospital, Tirupati during March 2016 to February 2017. The study was approved by the Institute’s Thesis Protocol Approval Committee (Letter Roc.No:AS/08/TPAC/SVIMS/2010, Dated:10-02-2016) and Institutional Ethical committee (Letter Roc. No. Roc.No.A&E/08/IEC/SVIMS/09, Dated: 29-02-2016). A written informed consent were obtained from all the study participants.
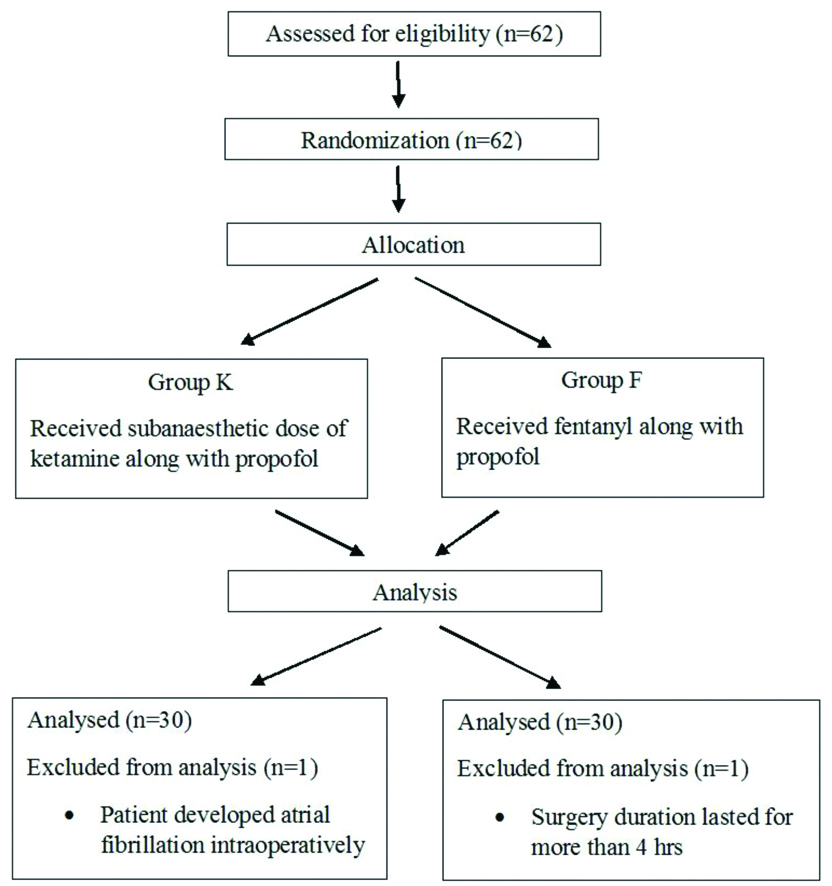
Sample size was derived using software openepi.com. For the sample size of the two groups, the power was set at 80% (β=0.2) and the significant level was set as 5% (α=0.05, two-tailed). The calculated sample size was minimum 56, so taking potential drop-outs into consideration; the sample size was set as 62 (31 in each group). Sixty-two American Society of Anaesthesiologists Physical Status (ASA) grade I and II adult patients (20-65 years of age) were randomised to receive either subanaesthetic dose of ketamine (Group K) or fentanyl (Group F) along with propofol. Randomisation was done by a computer generated random number table and sealed opaque envelope technique. Consort flowchart is depicted in [Table/Fig-1].
In all the patients selected for the study, a detailed pre-anaesthetic assessment was performed. They were explained about the study protocol and about 0-10 cm Numerical Rating Scale (NRS) to indicate their postoperative pain perception. Demographic data like age, sex, weight, Body Mass Index (BMI), ASA physical status grade were noted. They were kept nil by mouth 6 hours for solids and 2 hours for clear liquids and were received pre-medication with tablet alprazolam 0.5 mg and tablet ranitidine 150 mg orally on the night before surgery and on morning 2 hours before scheduled time of surgery.
On arrival at the operating room, an 18 gauge intravenous cannula was secured under local anaesthesia and routine monitoring were attached which included Non-Invasive Blood Pressure (NIBP), pulse oximetry (SpO2) and Electrocardiography (ECG). In addition, BIS (Covidien ireland ltd., Mansfield, MA, USA) and Train of Four (TOF) monitor (Fisher and Paykel healthcare Ltd., Auckland, New Zealand) were attached. Heart Rate (HR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial blood Pressure (MAP), SpO2, EtCO2 were noted at the baseline, before induction, before intubation, at 3,15,30,60 and every 30 minutes thereafter till the end of surgery. An 18 gauge epidural catheter was placed under LA at a dermatomal level appropriate for that surgery. Epidural test dose was given to rule out any intrathecal placement.
Group K received 0.5 mg/kg of inj.ketamine (Ketamax, Troikaa, India) I.V over 2-3 minutes, 15 minutes prior induction. Group F received the same volume of normal saline over the same period 15 minutes prior induction. Pre-oxygenation was done. Induction was done with inj.fentanyl (Verfen, Verve, India) 2 mcg/kg I.V and inj.propofol (Profol, Claris, India) at a rate of 30 mg/kg/hr infusion until the BIS value drops below 60. Tracheal intubation was facilitated with inj.vecuronium (Neovec, Samarth, India) 0.1 mg/kg i.v in both the groups and patients were intubated with appropriate sized cuffed endotracheal tube and were ventilated with (Drager fabius plus, Germany) 50% O2 in N2O with Volume Controlled mode of Ventilation (VCV). During maintenance, Group K received inj.ketamine 0.2 mg/kg/hr continuous i.v infusion whereas Group F received inj.fentanyl 1 mcg/kg/hr continuous i.v infusion. Propofol infusion rates in both the groups were titrated so as to maintain BIS around 40-60 and top up doses of inj.vecuronium 0.02 mg/kg i.v were given when the TOF count was ≥2.
Inadequate analgesia was defined as an increase in mean arterial pressure or HR by more than 20% of baseline values. When inadequate analgesia occurred with BIS within the recommended range (i.e., 40-60) [8], inj.fentanyl 0.5 mcg/kg i.v boluses were given. Hypotension was defined as drop in SBP or MAP by more than 30% of baseline or SBP below 90 mmHg was initially treated with 200 mL i.v fluid bolus, when not corrected then treated with inj. ephedrine (Efipres, Neon, India) 0.06 mg/kg i.v bolus. Bradycardia was defined as drop in pulse rate to less than 50 beats/min and was treated with inj. atropine (Anitrop, Montage, India) 0.6 mg i.v bolus.
All anaesthetic agents were discontinued 10 minutes before the anticipated end of surgery and total amount of propofol and vecuronium consumed were calculated. Residual neuromuscular blockade was reversed with inj.neostigmine (Neotagmin, Themis medicare, India) 0.05 mg/kg i.v and inj.glycopyrollate (Pyrolate, Neon, India) 0.01 mg/kg i.v when TOF count was >3. After meeting the criteria for extubation, patients were extubated and the time from discontinuation of anaesthetic agent to tracheal extubation was noted.
Postoperatively, Ramsay sedation score was assessed immediately after surgery and at 5, 15, 30 and 60 minutes thereafter until they achieve a score of 2. Time from completion of surgery to a Ramsay sedation score of 2 was recorded. All patients were visited in the recovery room and pain scores were evaluated using a 0-10 cm NRS (starting from 0 as no pain to 10- worst pain imaginable) every 15 minute till 1hour and then at 2, 4 and 8 hours after surgery or till NRS ≥4. When NRS ≥4, inj.tramadol 1.5 mg/kg i.v. bolus was given followed by activation of epidural infusion. Time from completion of surgery to first postoperative analgesic request (NRS ≥4) was recorded. They were monitored for any adverse cardiovascular and respiratory events, nausea, vomiting, shivering, excitation, euphoria, hallucinations or any other adverse events within 24 hours.
Statistical Analysis
All collected data were represented in excel chart and double checking was done for any clerical errors. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) software for windows version 21 (IBM SPSS Statistics v21.0, USA). The variability in data obtained was expressed either as median with inter-quartile range for nonparametric data or mean with standard deviation for normally distributed data. Proportions were reported with 95% confidence intervals. Continuous data was analysed with Student’s t-test or Mann-Whitney U test as appropriate. Categorical data was analysed with proportion, Chi-square test or Fisher’s-exact test as appropriate. A p-value less than 0.05 were considered statistically significant.
Results
Out of 62 patients who entered the study pool, two patients were excluded, one from each group. One patient developed atrial fibrillation intraoperatively and in the other patient surgery duration lasted for more than 4 hours.
[Table/Fig-2] reflects the demographic distribution between the groups. Both the groups were comparable with regard to age, weight, BMI, sex ratio and ASA physical status. Duration of surgery in both the groups was also comparable.
Comparison of demographic data between the study groups.
| Variables | Group K (n=30) | Group F (n=30) | p-value |
|---|
| Age (years) | 49.16±12.6 | 49.13±11.3 | 0.991* |
| Sex (Male/Female)(n) | 14/16 | 19/11 | 0.194** |
| Weight (Kg) | 52.26±8.37 | 55.86±9.35 | 0.122* |
| BMI (kg/m2) | 20.61±1.99 | 20.99±2.54 | 0.519* |
| ASA grade I/II (n) | 24/6 | 22/8 | 0.542** |
| Duration of surgery (min) | 201±25.3 | 198.0±21.4 | 0.623* |
Data entered as mean±Standard deviation; n=number of patients
p-values calculated using *Student’s t-test and **Chi-square test; p-value <0.05 considered statistically significant
[Table/Fig-3] shows intraoperative requirement of anaesthetic between the study groups. There was a statistically significant reduction in propofol requirement in propofol-ketamine group (p=0.011) but it was clinically insignificant. Total propofol consumed in propofol-ketamine group was 6.501±0.24 mg/kg/hr and in propofol-fentanyl group it was 6.672±0.26 mg/kg/hr.
Comparison of intraoperative requirements between study groups.
| Variables | Group K | Group F | p-value |
|---|
| Total propofol requirement (mg/kg/hr) | 6.501±0.24 | 6.672±0.26 | 0.011* |
| Total vecuronium requirement (mg/kg/hr) | 0.0692±0.004 | 0.0714±0.005 | 0.122* |
| No. of additional doses of fentanyl required (n) | 0 | 19 | 16 | 0.245** |
| 1 | 3 | 8 |
| 2 | 8 | 6 |
| Additional fentanyl required (mcg/kg/hr) | 0.088±0.123 | 0.099±0.115 | 0.734* |
Data entered as mean±Standard deviation; n=number of patients
p-values calculated using *Student’s t-test and **Chi-square test; p-value <0.05 considered statistically significant
Vecuronium was administered in both the groups to provide neuromuscular relaxation by maintaining TOF count <2. There was increased requirement of vecuronium in fentanyl group but it was statistically insignificant (p=0.122).
Patients in both the groups received additional doses of fentanyl as boluses of 0.5 mcg/kg i.v whenever there was inadequate analgesia. Eleven patients in propofol-ketamine group and 14 patients in propofol-fentanyl required additional fentanyl doses which were found to be statistically insignificant (p-value 0.245). Total amount of additional fentanyl required in both the groups were calculated which was found to be 0.088+0.123 mcg/kg/hr and 0.099±0.115 in propofol-ketamine group and propofol-fentanyl group, respectively (p-value 0.734).
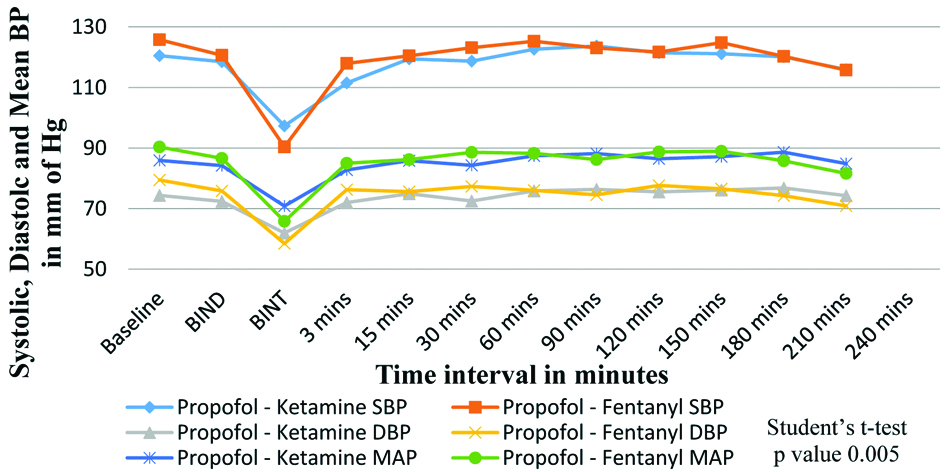
[Table/Fig-4] shows comparison of SBP, DBP and MAP (mm of Hg) between the two groups. There was a statistically significant fall in systolic and mean BP post-induction in fentanyl group (with mean SBP of 97.33±9.26 in Group K and 90.40±9.34 in Group F) with a p-value 0.005 and 0.018, respectively.
Comparison of systolic, diastolic and mean BP between the study groups.
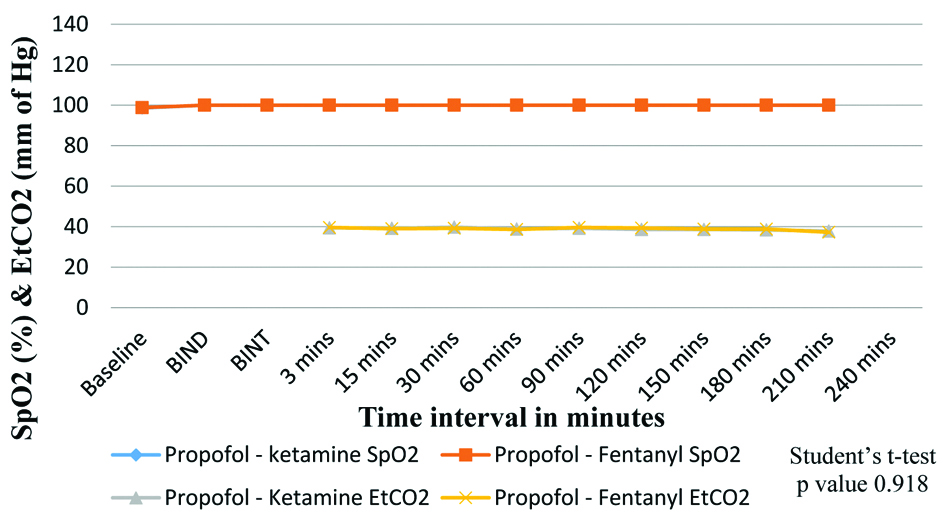
[Table/Fig-5] shows comparison of SpO2 and EtCO2 (mm of Hg) between the two groups. SpO2 and EtCO2 were not significantly different among the groups.
Comparison of SpO2 and EtCO2 between the study groups.
Group K=propofol-ketamine group, Group F=propofol-fentanyl group
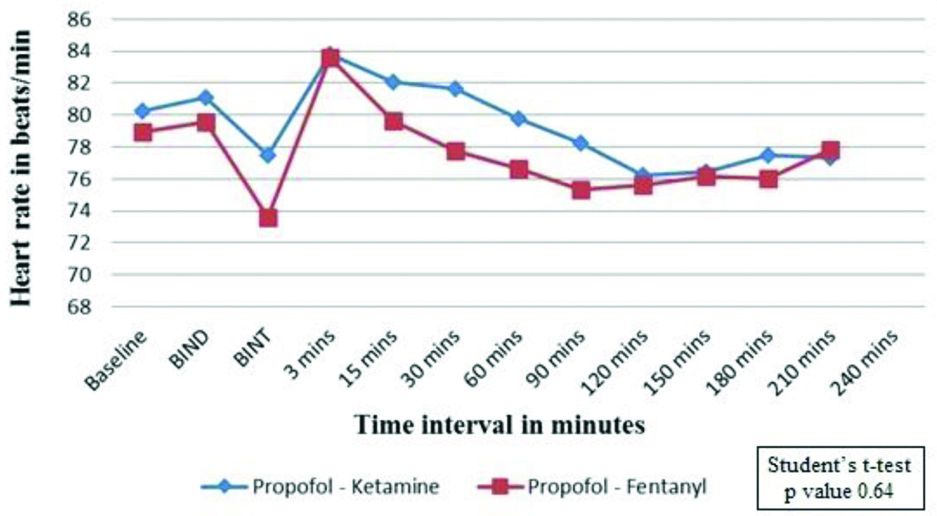
[Table/Fig-6] shows effect on HR (beats/min) between the two groups. Mean baseline HR in Group K was 80.23±11.70 and it was 78.96±8.98 in Group F with a p-value of 0.64. Post-intubation HR increased in both the groups which gradually decreased thereafter and reached baseline after 15 minutes. Although the changes in the HR were not significantly different, there was a trend towards a higher HR in propofol-ketamine group.
Comparison of Heart Rate (HR) between the study groups.
Group K=propofol-ketamine group, Group F=propofol-fentanyl group
[Table/Fig-7] compares recovery characteristics in the two groups. The time taken from discontinuation of anaesthetic agents to tracheal extubation was found to be 18.73±5.61 minutes and 16.23±6.80 minutes in propofol-ketamine group and propofol-fentanyl group, respectively. No significant difference was found between the two groups (p-value 0.126). Patients in propofol-ketamine group took little longer time (12.16±5.82 minutes) than those in propofol-fentanyl group (9.50±8.02) but the difference was statistically insignificant (p=0.146). There was also a significant delay in postoperative first analgesic request in propofol-ketamine group (57.50±38.20 vs 40.50±22.68 minutes in propofol-fentanyl group) (p-value 0.04).
Comparison of postoperative recovery profile between study groups.
| Variables | Group K (n=30) | Group F (n=30) | p-value |
|---|
| Time since anaesthetic discontinuation to tracheal extubation (min) | 18.73±5.61 | 16.23±6.80 | 0.126* |
| Time from end of surgery to Ramsay sedation score of 2 (min) | 12.16±5.82 | 9.50±8.02 | 0.146* |
| Time from end of surgery to first postop analgesic requirement (min) | 57.50±38.20 | 40.50±22.68 | 0.040* |
| Adverse events postoperatively | Yes | 7 | 5 | 0.519** |
| No | 23 | 25 |
Data entered as mean±Standard deviation; n=number of patients
p-values calculated using *Student’s t-test and **Chi-square test; p-value <0.05 considered statistically significant
Postoperatively, patients were monitored for any adverse cardiovascular or respiratory events, nausea, vomiting, shivering, excitation, euphoria, hallucinations or any other adverse events. Only two patients in propofol-ketamine group had psychomimetic effects, four patients had shivering and one had hypotension. Whereas, in fentanyl group five patients had hypotension.
Discussion
TIVA has been a subject of interest for all anaesthesiologists. It was initially attempted with a single drug (e.g., thiopentone, propofol) but was associated with significant side effects. Therefore, various drug combinations have been tried to produce desired results. Propofol, when used as a sole anaesthetic agent, a larger dose is required that may be associated with haemodynamic [9] and respiratory effects like hypotension, bradycardia, apnoea or hypoventilation [10]. Ketamine and opioids like fentanyl may be combined with propofol to decrease the above mentioned adverse effects.
The effects of TIVA are related to its plasma concentration. The inability to continuously monitor the plasma level of intravenous agents along with irretrievability of i.v agents add to the challenge of using a TIVA approach. Using BIS monitors, that has steadily gained clinical acceptance to monitor the effects of anaesthesia and sedation, the dose of hypnotic agents administered during maintenance phase can be minimised by titrating them accordingly.
Ketamine is known to increase blood pressure and HR when used in anaesthetic doses. This property is made use of when the anaesthesiologists used ketamine along with propofol while conducting TIVA. Though there was a haemodynamic stability, there was a problem of side-effects associated with ketamine infusion in high doses. So, it was attempted to prove whether the subanaesthetic dose of ketamine propofol mixture is any way superior to standard TIVA technique using propofol-fentanyl combination.
The present study was done to evaluate the effects of fentanyl or subanaesthetic dose of ketamine when given along with propofol on intraoperative anaesthetic requirement, postoperative analgesia and recovery profile in patients receiving TIVA. It was observed that immediately after induction, haemodynamics were well maintained in the ketamine group than in fentanyl group which was found to be statistically significant. But postintubation, there were only minimal changes in blood pressures among the two groups recorded at various time intervals which were statistically insignificant. Although the changes in HR were not significantly different, there was a trend towards a higher HR in the ketamine group.
Bajwa S and Kaur J, found a decrease in HR in propofol-fentanyl group postinduction which returned gradually towards baseline during maintenance phase and a statistically significant higher HR in propofol-ketamine group which persisted until 5 minutes postsurgery [7]. They also observed a statistically significant fall in SBP postinduction in the propofol-fentanyl group and a slight increase in blood pressure in propofol-ketamine group. Mahajan R et al., found a statistically significant fall in SBP after induction in propofol-fentanyl group [11]. Pawar D et al., found a significant increase in HR in ketamine group when compared with pre-induction values which continued till 10 minutes postinduction [12]. Also, they observed a minimal rise in postinduction SBP after 5 minutes in ketamine group.
Similar findings were observed in this study where a statistically significant fall in SBP occurred in propofol-fentanyl group postinduction. The initial beneficial effect of non-lowering of blood pressure postinduction in propofol-ketamine group may be due to bolus administration of ketamine 0.5 mg/kg given prior to induction. Propofol decreases arterial pressure in healthy patients by decreasing peripheral vascular resistance and myocardial contractility. Ketamine produces dose-related increases in the rate-pressure product and a transient increase in cardiac index, so that both peripheral resistance and HR are augmented. When used along with propofol, the cardio-stimulant effect of ketamine, even in subanaesthetic doses, may balance the cardio-depressant pressure effects of propofol [13]. This study differs from all other studies that a sub-anaesthetic dose of ketamine (0.2 mg/kg/hr) was used as intraoperative infusion so as not to affect the BIS which was used to monitor the depth of anaesthesia [14].
A minimal but statistically significant reduction in propofol requirement was observed in ketamine group (p=0.011). Sharma R et al., also observed an increase in consumption of propofol in propofol-fentanyl group (164 mg versus 148 mg in propofol-ketamine group) [15]. But Akhondzadeh R et al., observed no significant difference between the two groups for additional requirement of propofol [16]. This may be because the procedure done was Endoscopic Retrograde Cholangio Pancreatography (ERCP) which was minimally painful. Messenger D et al., observed that, the cumulative dose of propofol to maintain sedation was higher in ketamine group (1.5±0.9 mg/kg versus 1.1±0.6 mg/kg in propofol-fentanyl group) [17]. This may be due to the fact that only a bolus dose of 0.3 mg/kg ketamine was given which might have been insufficient with respect to duration of action and comparative dosing with that of fentanyl 1.5 mcg/kg.
In this study, although there was a statistically significant reduction in dose of propofol required in ketamine group, it was clinically insignificant (6.501±0.24 mg/kg/hr in ketamine group vs 6.672±0.26 mg/kg/hr in fentanyl group) with a p-value 0.011. An initial bolus of 0.5 mg/kg ketamine was given followed by continuous infusion (0.2 mg/kg/hr) during maintenance. This might have led to decrease in dose of propofol required to maintain effective depth of anaesthesia (i.e., BIS around 40-60). If a larger dose of ketamine was used as in other studies a clinically significant reduction in propofol requirement may be observed as well.
There was no statistically significant difference between the two groups with respect to intraoperative vecuronium requirement. But there was an increased requirement for vecuronium in both the groups (0.0692±0.004 mg/kg/hr in propofol-ketamine group and 0.0714±0.005mg/kg/hr in propofol-fentanyl group). This might be due to lack of additive effect of intravenous anaesthetics on reducing muscle relaxants.
Bajwa S and Kaur J, assessed recovery profile using Stewards Scoring System, (which evaluates ventilation, movement and wakefulness) [7]. They observed that movement score and wakefulness score were better in fentanyl group. They theoretised that it was due to the prolonged sedative effects of ketamine which might have led to the late return of wakefulness and voluntary movements. They also observed that, spontaneous recovery as measured by the return of protective airway reflexes in response to verbal commands was achieved earlier in fentanyl group than the ketamine group. Ramdev B et al., assessed recovery profile by Modified Steward Coma Score (score of six was an index for adequate recovery) observed that, recovery was better in fentanyl group (25% patients in ketamine group had a score of six at 15 minutes postsurgery while 30% patients in fentanyl group had a score of six at 5 minutes postsurgery) [18].
The above studies were done in patients who underwent ambulatory day care surgeries lasting less than one hour duration. But this study was conducted in surgeries lasting for a maximum of four hours duration. So, Ramsay sedation score was used to assess the recovery of the patient. A Ramsay sedation score of 2 is set as an index for adequate recovery. In our study, though there was a two and a half minute delay in recovery in patients receiving propofol-ketamine, it was not statistically significant. This might be due to the fact that in this study a lower dose of ketamine was used.
Ketamine at anaesthetic doses may cause various emergence phenomena like hallucinations, nightmares and delirium. At sub-anaesthetic doses (0.1-0.5 mg/kg), it produces anti-nociception, but may be associated with cognitive, perceptual, mood disturbances, and psychomimetic side-effects. The incidence of psychomimetic effects was small when ketamine was combined with propofol for general anaesthesia or sedation [19]. In this study, two patients (6%) had psychomimetic side-effects which was manifested as either excitation, euphoria or hallucinations. They were self-limiting and patients were relieved without any medications within 20-30 minutes. Though a low dose of ketamine (0.2 mg/kg/hr) was used in this study, the infusion duration was comparatively longer (approx. 3½ hrs) and the infusion was continued till 10 minutes before the anticipated skin closure. These might have resulted in excitatory side effects.
Several investigators [20-22] reported a decrease in psychomimetic emergence reactions when ketamine was used in combination with a sedative-hypnotic (e.g., benzodiazipines, propofol). Sub-hypnotic doses of propofol may fail to block ketamine induced hallucinations [23]. However, hypnotic doses of propofol are reported to block these hallucinations [24,25]. Hui T et al., calculated the ED95 for hypnosis in propofol-ketamine combination to be 0.97 mg/kg propofol and 0.33 mg/kg ketamine [26]. In this study, mean total dose of propofol-ketamine combination used for both induction and maintenance was 6.5 mg/kg/hr propofol and 1.17 mg/kg ketamine.
It was observed that, patients in ketamine group had a significant delay in requirement for first post-operative analgesia (57.5±38.2 vs 40.5±22.6 min with p-value 0.04). Ketamine, an NMDA antagonist modifies the induction of central sensitisation and contributes significantly to prevention of pain. It also suppresses visceral pain sensitisation. It probably reduces both peripheral and central, visceral NMDA and non-NMDA receptor nociception and enhanced pain inhibiting systems such as monoaminergic mechanisms. In this study, only a brief period of anti-nociception was observed postsurgery because only 0.2 mg/kg/hr of ketamine infusion was given, which was not continued post-surgery.
Limitation(s)
Long-term sequelae of ketamine were not observed. Ketamine infusion was not continued postoperatively.
Conclusion(s)
Pre-inductional bolus (0.5 mg/kg) of subanaesthetic ketamine followed by infusion (0.2 mg/kg/hr) intraoperatively maintains haemodynamic stability, provides analgesia, decreases anaesthetic requirement and delays the request for first post-operative analgesia. Hence, addition of subanaesthetic ketamine bolus and an infusion was suggested in patients receiving TIVA.
Data entered as mean±Standard deviation; n=number of patients
p-values calculated using *Student’s t-test and **Chi-square test; p-value <0.05 considered statistically significant
Data entered as mean±Standard deviation; n=number of patients
p-values calculated using *Student’s t-test and **Chi-square test; p-value <0.05 considered statistically significant
Data entered as mean±Standard deviation; n=number of patients
p-values calculated using *Student’s t-test and **Chi-square test; p-value <0.05 considered statistically significant