Abdominal hysterectomy, one of the most commonly performed major gynaecologic surgeries, is associated with medium to high levels of postoperative pain [1]. Inadequate perioperative analgesia can have various immediate and long-term consequences often necessitating increased length of hospital stay due to delayed recovery. There is also a possibility of delayed consequences in the form of chronic pain syndromes (posthysterectomy chronic pain syndrome) [2]. Moreover, patients’ satisfaction and subjective success of the operation are crucially influenced by the efficacy of analgesia, both in the intraoperative and postoperative period.
Various analgesic regimens have been in use for postoperative analgesia in abdominal hysterectomy. Buprenorphine is a semi synthetic derivative of thebaine, a morphine alkaloid; a potent and safe analgesic when compared to that of morphine. It has a strong agonistic activity at the μ-receptor and antagonistic properties at the κ receptor. Its high lipid solubility makes it highly effective through the transdermal route. It is a powerful analgesic, approximately 75-100 times as potent as morphine in transdermal formulation and causes less respiratory depression [3]. It also has a long half-life with relatively less side-effects. Its safety and usefulness in chronic pain has been well documented but limited literature exists on its role in postoperative pain in gynaecological surgery [4]. Epidural administration of analgesics as continuous infusion through an epidural catheter is one of the most widely used methods to provide postoperative analgesia in gynaecological surgeries [5]. Various drug combinations using local anaesthetics and adjuvants including opioids have been tried in epidural infusions.
A relatively novel approach in postoperative analgesia includes the use of Transdermal Drug Delivery Systems (TDS). Buprenorphine is homogeneously incorporated in solid matrix patch which is applied to skin. It is available as three types of preparations. The commonly used ones are high dose (35 mcg/h, 52.5 mcg/h, or 70 mcg/h) patches kept for three days and low dose patches (5 mcg/h, 10 mcg/h, or 20 mcg/h) for 7 days [6]. TDS provides safe, convenient and sustained method of delivery. The numerous advantages include non-invasive administration, sustained drug delivery precluding occurrence of break through pain, better bioavailability (less first pass metabolism) and lesser adverse effects [7]. Transdermal preparations have been in use for the past few years in diverse acute and chronic pain syndromes particularly cancer pains and have been found to be safe even in elderly [3]. Surprisingly, TDB has not gained much popularity in postoperative analgesia protocols till recently and very few studies have explored its use in gynaecological surgeries [4]. TDB patch (10 mg) has been found to be safe and effective in postoperative pain following lower limb and spine surgeries [8-10]. It was also found to be reduced the postoperative rescue analgesic requirements and provide better haemodynamic stability in patients undergoing major abdominal surgery [11]. However, there is not much literature comparing the use of TDB to the time-tested epidural route [12].
The aim of this study was to compare the analgesic efficacy of TDB with epidural buprenorphine infusion in postoperative pain relief following abdominal hysterectomy. Postoperative NRS and requirement of rescue analgesics was used as outcome measures. The secondary objectives included identifying the incidence of various adverse effects including nausea, vomiting, respiratory depression, pruritus and sedation.
Materials and Methods
A prospective observational cohort study was undertaken on 116 patients posted for elective abdominal hysterectomy in the study institution, between January 2017 to March 2018. The Institutional Ethics Committee approved the study (GMCKKD/RP2017/IEC/131).
Inclusion criteria: Patients enrolled were between the age of 30 to 65 years, belonging to American Society of Anaesthesiologists’ (ASA) Physical Status (PS) I-II and weighed between 45 to 65 kg.
Exclusion criteria: Those having known hypersensitivity to opioids or patch additives, Chronic Obstructive Pulmonary Disease (COPD)/obstructive sleep apnoea, psychiatric disorders or those with chronic pain syndromes were excluded. In addition, patients whose surgical procedure extended for more than two hours were also subsequently excluded from the study.
Informed written consent was obtained from all the enrolled patients. After enrolment, study participants were divided into two equal groups of 58 each, using random number table. The patients were instructed about the study drugs, NRS and postoperative pain treatment options, a day before surgery. All patients were premedicated on the night before surgery with ranitidine 150 mg and metoclopramide 10 mg orally. Patients were kept in fasting for 8 hours prior to surgery.
Group T patients received TDB patch 10 μg/h placed 12 hours prior to surgery (effective serum concentrations for TDB is achieved after 12-24 h) [3]. The patch was applied to hair-less sites most commonly at upper arm, side of chest or upper back.
Niyogi S et al showed that a TDB patch (10 μg/hour) applied 24 hours before surgery can be used as postoperative analgesic for lumbar fixation surgery [7]. It reduced postoperative rescue analgesic consumption over 48 hours and maintained haemodynamic stability without serious complications like respiratory depression, sedation or Postoperative Nausea and Vomiting (PONV). So, in this study, 10 μg/h TDB patch in Group T was chosen.
On the day of surgery, baseline parameters including Heart Rate (HR), Blood Pressure (BP), Oxygen Saturation (SpO2) and sedation scores were recorded. Intravenous (IV) line was established, normal saline infusion was started and injection ondansetron 4 mg IV was given as a prophylactic dose to prevent PONV.
Epidural puncture was done at L1-L2 or L2-L3 space for patients in Group E; 18 G epidural catheter was passed and kept in place. Surgery was done under subarachnoid block with 3.4 mL 0.5% bupivacaine heavy along with 60 μg buprenorphine in all patients. All patients received supplemental O2 with simple face mask at 5 L/min.
After establishing adequate level of blockade, surgery was started and all the vital parameters monitored continuously. At the end of surgery, patients were shifted to recovery room and carefully observed. For patients in Group E epidural infusion with Buprenorphine 10 μg/h was immediately commenced on shifting to recovery room.
In the postoperative period, the patients were monitored for 72 hours. Postoperative analgesia was assessed using NRS and the requirement of rescue analgesic. It consists of a scale from 0 to 10 where 0 represent “no pain” and 10 the “worst imaginable pain.” Patients were asked to indicate the strength of pain in this scale verbally. The time interval of monitoring was as follows-0 hour, 2 hours, 4 hours, 24 hours, 36 hours, 48 hours, 60 hours and 72 hours after surgery (the time at which the patient arrived in the recovery room was taken as time 0 hour). If the NRS value was >4, Inj. diclofenac 75 mg IV to a maximum of two doses over 24 hours was given. Inj. paracetamol 1 gm IV infusion to a maximum of three doses was given as second line rescue analgesic. The requirement of rescue analgesics was also recorded every 24 hours.
All the patients were monitored for any side-effects like sedation, nausea, vomiting, respiratory depression, pruritus, headache, dizziness, patch site redness etc. Vomiting and nausea were treated with injection Ondansetron 4 mg IV. Ramsay Sedation Scale (RSS) was used to assess sedation on arrival in the operating room and postoperatively at regular intervals [13].
Haemodynamic parameters were also monitored during the postoperative period and managed if necessary. The occurrence of respiratory depression with Respiratory Rate (RR) <8/min or SpO2 <95% was carefully watched for and managed with appropriate respiratory support.
The transdermal patch and the epidural catheter were removed after 72 hours postoperatively or alternatively the patch was continued for two days more.
Statistical Analysis
Keeping alpha error at 0.05 and 90% power, it was estimated that 58 patients were required per group to achieve statistical significance (p-value <0.05). All the raw data were entered into Microsoft Excel spreadsheet and statistical analysis of data was done using Statistical Package for the Social Sciences (SPSS) software version 18. Qualitative data like ASA PS, adverse effects were compared using Chi-square test. Quantitative data like NRS, RSS were compared using Independent t-test and requirement of rescue analgesics using Wilcoxon ranksum test. Data are presented as mean±standard deviation, median (IQR) or as the number of patients and percentages. The p-value <0.05 was taken as statistically significant.
Results
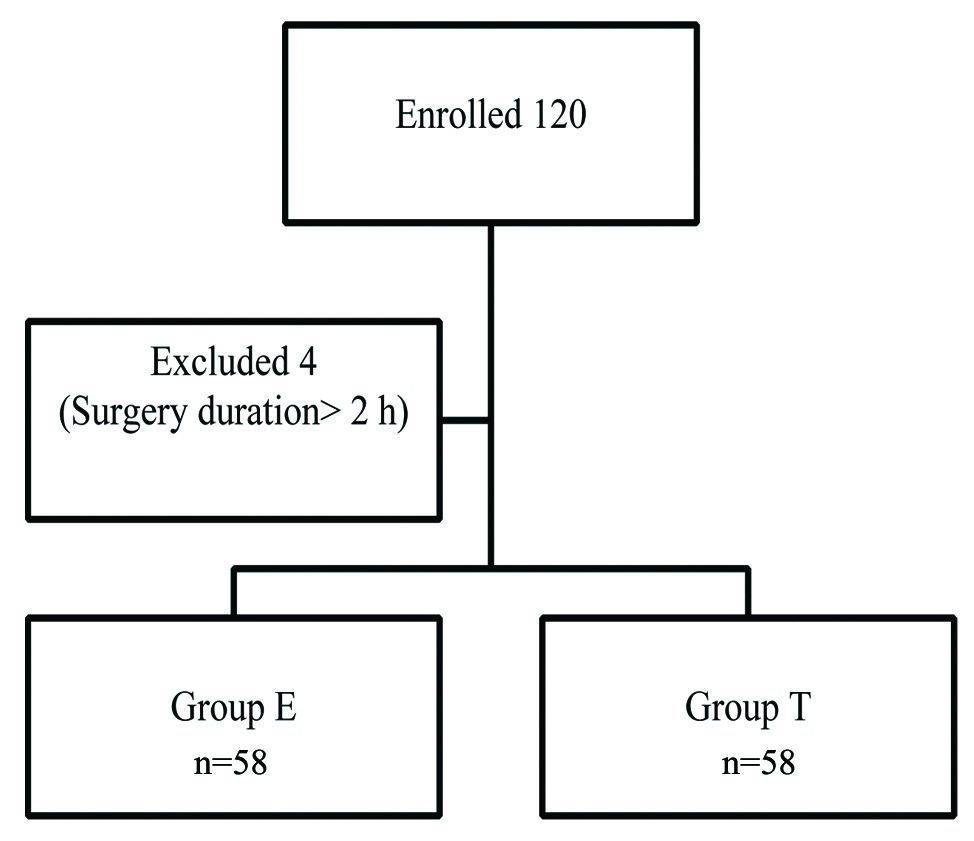
A total of 120 patients were enrolled in the study, of which four were excluded from analysis as their surgical procedure extended to beyond two hours. Hence, a total of 58 patients in each group were included for the final analysis [Table/Fig-1].
The two groups were comparable in terms of demographic parameters such as age, weight and ASA physical status [Table/Fig-2]. The degree of analgesia in two groups were assessed by NRS at 0 hour, 2 hours, 4 hours, 24 hours, 36 hours, 48 hours, 60 hours and 72 hours postoperatively. At all points of measurements pain scores were more with transdermal group and all were statistically significant [Table/Fig-3].
Comparison of demographic and baseline characteristics.
| Characteristics | Group E (n=58) | Group T (n=58) | p-valueUnpaired t-test*Chi-square test† |
|---|
| Age (Years)* | 47.43±4.57 | 47.67±4.64 | 0.95 |
| Weight (kg)* | 63.43±5.58 | 64.07±3.8 | 0.90 |
| ASA Physical status† |
| I | 34 (58.6) | 36 (62.1) | 0.42 |
| II | 24 (41.4) | 22 (37.9) |
*Data represented as mean±SD; †number of patients, Chi-square test n (%); ASA: American society of anaesthesiologists; SD: Standard deviation
Comparison of Numerical Rating Scale (NRS) scores.
| Time point (Hour) (Postoperative) | Group E (n=58) | Group T (n=58) | p-value(Independent t-test) |
|---|
| 0 | 0 | 0 | |
| 2 | 1.24±1.38 | 3.10±1.03 | <0.001 |
| 4 | 2.58±1.22 | 3.86±0.86 | <0.001 |
| 24 | 3.50±0.82 | 4.74±0.71 | <0.001 |
| 36 | 3.51±0.88 | 4.54±0.75 | <0.001 |
| 48 | 3.10±0.89 | 4.15±0.85 | <0.001 |
| 60 | 2.63±0.98 | 3.46±0.84 | <0.001 |
| 72 | 1.31±1.27 | 2.31±0.99 | <0.001 |
Data represented as mean (Standard Deviation)
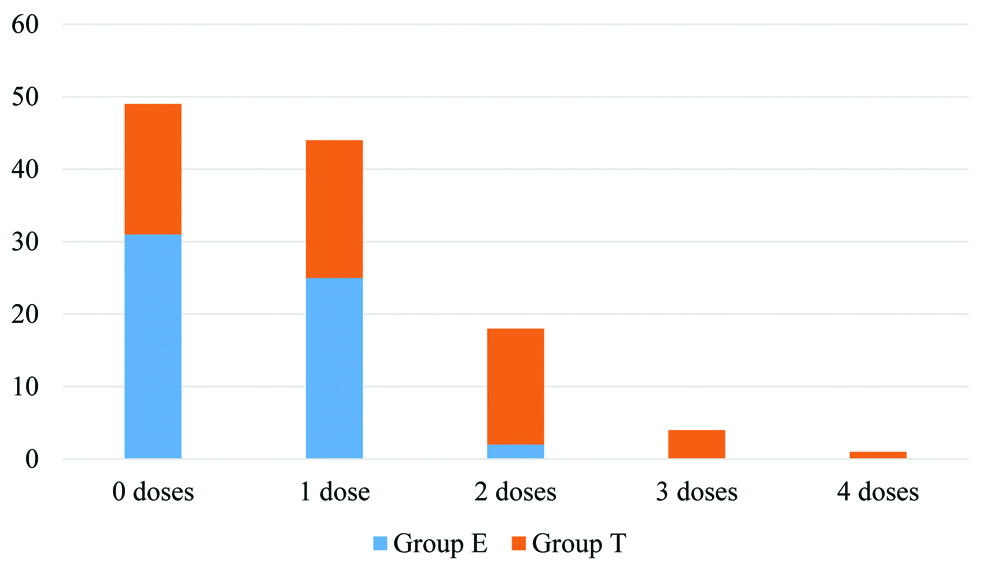
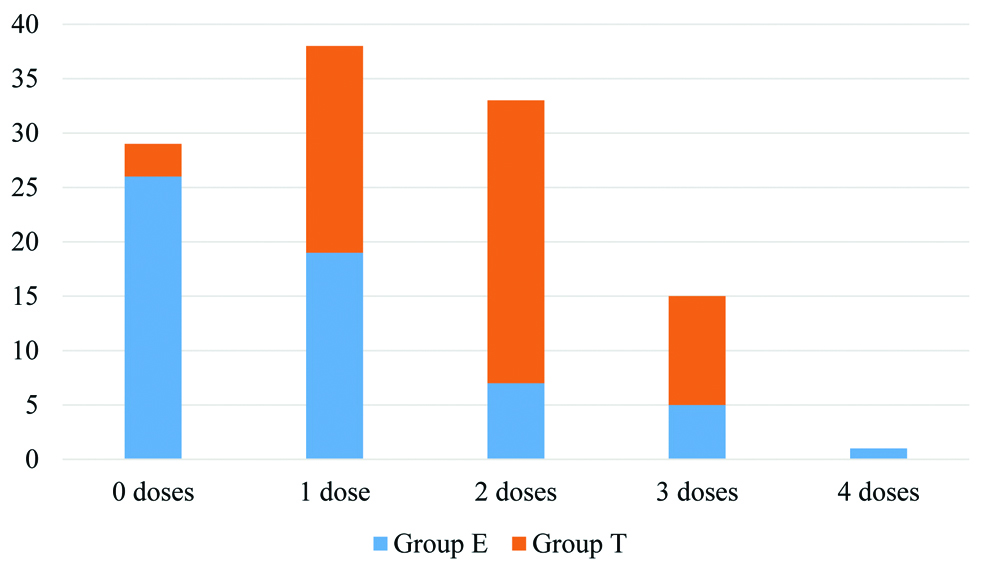
The requirement of paracetamol and diclofenac given intravenously as rescue analgesics was compared between the two groups in 24-hour intervals throughout the study period. The requirement of paracetamol (second line rescue analgesic) was found statistically higher in the first 48 hours and requirement of diclofenac was significantly higher in 48 to 72 hours for group T [Table/Fig-4]. The median dose (IQR) of diclofenac required was 0 (0, 75) in Group E and 75 (0, 150) in Group T while that of paracetamol was 1 (0, 1) in Group E and 2 (1,2) in Group T. Both were significantly higher in Group T [Table/Fig-5,6]. The number of patients requiring rescue analgesics was also higher in Group T than Group E. Of the 58 patients in Group E, 21 (36.2%) did not require any rescue analgesic, 19 (32.75%) required twice and the rest 18 (31.03%) required a single dose of rescue analgesic. In Group T, 50 (86.2%) patients required multiple doses and only 1 (1.72%) did not require any rescue analgesic in the entire study period.
Comparison of rescue analgesic requirement.
| No. of doses | Group E (n=58) | Group T (n=58) | p-value (Wilcoxon ranksum test) |
|---|
| First 24 hours for Inj Paracetamol |
| 0 | 31 | 11 | <0.001* |
| 1 | 24 | 39 |
| 2 | 3 | 8 |
| Median (IQR) | 0 (0, 1) | 1 (1, 1) |
| First 24 hours for Inj Diclofenac |
| 0 | 41 | 42 | 0.98 |
| 1 | 17 | 13 |
| 2 | 0 | 3 |
| Median (IQR) | 0 (0, 1) | 0 (0, 1) |
| 24-48 hours Inj Paracetamol |
| 0 | 43 | 19 | <0.001* |
| 1 | 15 | 38 |
| 2 | 0 | 1 |
| Median (IQR) | 0 (0, 1) | 1 (0, 1) |
| 24-48 hours Inj Diclofenac |
| 0 | 46 | 26 | <0.001* |
| 1 | 12 | 31 |
| 2 | 0 | 1 |
| Median (IQR) | 0 (0, 0) | 1 (0, 1) |
| 48-72 hours Inj Paracetamol |
| 0 | 51 | 52 | 0.77 |
| 1 | 7 | 6 |
| 2 | 0 | 0 |
| Median (IQR) | 0 (0, 0) | 0 (0, 0) |
| 48-72 hours Inj Diclofenac |
| 0 | 58 | 44 | <0.001* |
| 1 | 0 | 13 |
| 2 | 0 | 1 |
| Median (IQR) | 0 (0, 0) | 0 (0, 0) |
* p-value significant; IQR: Interquartile range
Total number of diclofenac doses.
Total number of paracetamol doses.
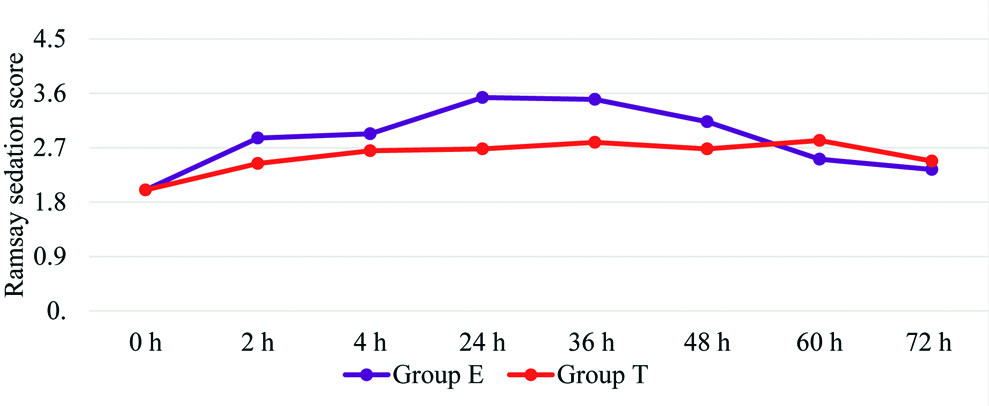
Sedation as assessed by Ramsay sedation score was higher with Group E than Group T [Table/Fig-7]. There was a significantly higher incidence of PONV among Group T patients. The antiemetic requirement was also concomitantly higher in Group T than Group E [Table/Fig-8].
Comparison of Ramsay Sedation Scale (RSS).
Independent t-test, p-values 2h:0.01, 4h:0.13, 24h:<0.001, 36h:<0.001, 48h:0.02, 60h:0.07, 72h:0.36
Comparison of Postoperative Nausea and Vomiting (PONV).
| Group | No. of episodes of PONV* | p-value(Chi-square test) |
|---|
| 0 | 1 | 2 |
|---|
| Group E | 50 (86.2%) | 8 (13.8%) | 0 | <0.001 |
| Group T | 26 (44.8%) | 26 (44.8%) | 6 (10.3%) |
Data given as number of patients (%); p-value <0.05 considered significant
All the patients in both groups maintained stable haemodynamics throughout the study period with two single episodes of hypotension in Group T which did not require any treatment. There were no significant episodes of bradycardia or respiratory depression in either of the two groups. None of the study patients complained of pruritus, headache, giddiness or patch site redness.
Discussion
Buprenorphine is a versatile drug, which has established a firm footing in the clinical setting of postoperative analgesia. Though it has been documented to be effective and safe in postoperative analgesia by the epidural as well as transdermal routes, both these modalities have not been extensively studied in comparison [4,5,7,12,14]. The present study compared the efficacy and safety of TDB (10 μg/h) with epidural infusion of buprenorphine at 10 μg/h and it was observed that epidural buprenorphine was more effective in obviating postoperative pain following abdominal hysterectomy as evidenced by lower NRS scores and lesser rescue analgesic requirement. A recent study by Rajan S et al., compared postoperative analgesia in major abdominal surgeries between patients receiving 10 mg TDB placed 24 hours pre operatively and single bolus dose of epidural buprenorphine (150 μg) and inferred that epidural buprenorphine was superior to transdermal [12]. They also observed that the NRS scores became comparable in both groups after 30 hours and they attributed this to the onset of action of the transdermal preparation.
In the present study, the peak NRS scores were recorded at 24 hours and it showed considerable reduction only after 48 hours probably corresponding to the onset time of the patch. The NRS scores never achieved comparable levels because the epidural group received continuous buprenorphine infusion, as opposed to single bolus in the study by Rajan S et al., [12]. The observed superiority of epidural buprenorphine may in part be explained by the fact that epidurals deliver drugs directly to the target nerve roots [12]. This combined with the high lipophilic nature of buprenorphine facilitates rapid absorption through epidural veins, reaching the brain thus producing rapid onset and longer duration of analgesia. Moreover, transdermal drug delivery may additionally be influenced by factors like skin age, hydration of skin, temperature and pH of skin and blood flow through the skin affecting drug absorption from the site of application of patch [15].
Timing of TDB patch application has been found to be a critical factor in its efficacy. Desai SN et al., in an Randomised Control Trial (RCT) on patients undergoing hip fracture surgeries found that a 10 μg/h patch applied 24 hours before surgery resulted in lower pain scores and lower rescue analgesic requirement (68% patients) when compared to those receiving oral tramadol [16]. Another study by Kadapamannil D et al., comparing analgesic efficacy with TDB (10 μg/h) placements 48 and 72 hours prior to major abdominal surgery, showed that NRS at 24 hours and 48 hours was two and one in the two groups [17]. In the present study NRS in group T at both 24 hours and 48 hours was >4. This may be because the patch was applied only 12 hours before surgery. It was reported that the onset of peak action following transdermal application of buprenorphine could take 48-72 hours. This is the main disadvantage of transdermal preparations affecting the onset of drug action and can present a potential limitation when drug delivery has to be rapidly modulated to changing requirements [4]. But placing the patch early in the absence of pain stimulus has many implications, as there is possibility of excessive sedation, respiratory depression, nausea, and vomiting once the peak action time starts and hence would mandate strict monitoring. Moreover, there were few logistical issues involved as the final surgical list gets decided only later in the day before surgery. Hence, a more conservative approach was opted for and the patch was placed 12 hours prior to the surgery.
Varying dosages of TDB patches have been studied in literature. Setti T et al., used 17.5, 35, 52.5 mg TDB patches placed 12 hours before in patients posted for open gynaecologic surgeries [4]. They found analgesic efficacy to be proportional to increasing doses with comparable side-effects in all groups. Few other researchers have also studied postoperative analgesia with higher doses of TDB (20 mg, 35 mg) and observed similar results [11,18,19]. Successful postoperative analgesia has been demonstrated in many studies using 10 μg/h TDB [7,16,17] But, a comparable efficacy to epidural buprenorphine could not be demonstrated in this study using 10 mg TDB. Further studies using a higher dose of the patch is advisable in the future to demonstrate a favourable analgesic profile in comparison to epidural. Setti T et al., noticed from their observations that TDB alone may not have been sufficient for postoperative analgesia, but always needed concurrent analgesic modalities [4]. Postoperatively, regular intramuscular doses of diclofenac were given in the study groups along with patch placement in another study by Niyogi S et al., and found a significantly lower rescue analgesic requirement compared to the placebo patch group [7]. The rescue analgesia requirement in the present study was higher in the TDB group, but showed a decrease after 48 hours corresponding to the peak action of TDB.
There was a significantly higher incidence of PONV and increased antiemetic use in TDB group. This is contrary to what was observed in most of the studies using a similar dose of TDB [7,16]. It has been documented that postoperative pain especially visceral or pelvic pain is a common cause of PONV [20-22]. The high incidence of PONV in this study correlated with the higher postoperative pain scores in patients receiving TDB. Furthermore, open major gynaecological surgery has been known to be associated with a PONV risk as high as 58% [23].
Apart from PONV, no other adverse effects including excessive sedation were seen with buprenorphine in both the groups. Similar studies using 10 mg patch have not documented sedation as a significant adverse effect [7,16]. Of note, buprenorphine did not produce any serious opioid related adverse effects like respiratory depression or bradycardia. Pergolizzi J et al., have described that buprenorphine, unlike other opioids, has full μ-agonist analgesia with no ceiling effect, at the same time clearly demonstrating a ceiling effect for respiratory depression [24]. So, its safety profile is well supported for postoperative use.
Limitation(s)
One of the main drawbacks of this study was that transdermal drug delivery was compared to a neuraxial technique in the same dose of study drug. Estimation of plasma concentration of buprenorphine could have helped to arrive at an equipotent dose for routes with varying bioavailability. Earlier patch application, though was not possible in this study, would have improved the analgesic efficacy, so that peak plasma levels of buprenorphine would correspond to the higher pain scores noted between 24 to 36 hours. Future studies could further explore patient satisfaction as an independent outcome variable, as this would help to better define the role of TDB in postoperative analgesia.
Conclusion(s)
Epidural buprenorphine given as continuous infusion is safe and more effective than TDB in an equal dosage in reducing postoperative pain in abdominal hysterectomy. Even though epidural buprenorphine given as continuous infusion was found to be more effective, TDB will find its role in postoperative analgesia as a component of multimodal pain management. Proper selection of dose and timing of application would be critical in its efficacy.
*Data represented as mean±SD; †number of patients, Chi-square test n (%); ASA: American society of anaesthesiologists; SD: Standard deviation
Data represented as mean (Standard Deviation)
* p-value significant; IQR: Interquartile range
Data given as number of patients (%); p-value <0.05 considered significant