There is a need to identify a score that measures the presence of organ dysfunction and its severity in children with sepsis and severe sepsis as per old and new definitions respectively. Of the several scoring systems to measure multi organ dysfunction in paediatrics, the Paediatric Sequential Organ Failure (PELOD) has been used most frequently [10-12]. The score was upgraded and validated to PELOD-2 in 2013 based on data from nine PICUs in France and Belgium. PELOD-2 score has shown good discrimination for in house mortality in sick children admitted to PICU [9,11,13-15]. However, this validation needs to be verified in other geographical areas before using this score in those places [12]. The first validation study of PELOD-2 from India (Maharashtra) was published recently which concluded good prediction of mortality by day 1 PELOD-2 scores in overall PICU admissions [16].
Study was conducted to evaluate the performance of PELOD-2 scores in predicting the outcome in the subset of “severe sepsis” patients admitted to PICU of a Tertiary Care Centre in North India, and to infer whether it can be proposed to revise the diagnostic criteria and paediatric sepsis definitions.
Materials and Methods
This prospective cohort study was conducted from October 2018 to September 2020 at the 8 bedded PICU of Department of Paediatrics, Jawaharlal Nehru Medical College and Hospital, Aligarh, Uttar Pradesh, India. The study was approved by the Institutional Ethics Committee (D.No.246/FM).
Inclusion and Exclusion criteria: The consecutive patients of severe sepsis aged 1 month to 14 years admitted to the PICU were included in the study. An informed written consent was taken from parents. Patients deceased or transferred to other health facility within 24 hours of admission, who underwent surgery in first 24 hours of hospitalisation, or those admitted post Cardio-Pulmonary Resuscitation (CPR) or whose parents did not consent were excluded from the study.
The diagnosis of severe sepsis was made by the attending physician in the PICU, based on the 2005 consensus definition of presence of SIRS along with infection and organ dysfunction [4].
The baseline parameters recorded were age, gender, and diagnosis at the time of PICU admission, length of PICU stay and outcome in terms of mortality or discharge. For PELOD-2 scoring, five organ systems (neurologic, cardiovascular, respiratory, renal, and haematologic) were considered and 10 variables (Glasgow Coma Scale (GCS), pupillary reaction, lactatemia, mean arterial blood pressures, PaO2/FiO2 ratio, PaCO2, invasive ventilation, creatinine, white blood cell count, and platelets) were recorded.
The PELOD-2 scoring was done within the first 24 hours. If a variable was measured more than once in 24 hour, the worst value was used in calculating the score. The maximum points for each organ dysfunction range between 2 to 10 and the overall maximum score is 33 [12,16].
Statistical Analysis
The data analysis was done by Statistical Package for the Social Sciences (SPSS) version 20.0. Quantitative data was expressed in mean, Standard Deviation (SD), median, Inter-Quartile Range (IQR). Independent t-test and Mann-Whitney U tests were used to analyse quantitative data, depending on the distribution of variables by the Kolmogrov-Smirnov test. The qualitative data was expressed in frequencies and percentages and Chi-square test was used for analysis.
The Standardised Mortality Ratio (SMR) was calculated from scores. The discriminatory power of the score was assessed by area under ROC (AUC with 95% confidence interval) and the calibration of the score was assessed by Hosmer-Lemeshaw goodness of fit test, with acceptable calibration at p>0.05. Youdens J statistic was used to define best cutoffs for sensitivity and specificity for predicting mortality by PELOD-2 scores.
Results
A total of 216 patients of severe sepsis were admitted consecutively to PICU during the study period, out of which 203 patients met the inclusion criteria. Thirteen patients were excluded from study (6 died within 24 hours, 4 left the hospital for another hospital and in 3 cases, the data was incomplete).
The mean age was 37.87 (±46.97 SD) months and 51.7% of the total patients were males. At the time of PICU admission, 86 (42.36%) patients were in septic shock, and 68 (33.49%) patients had Acute Respiratory Distress Syndrome (ARDS) at PICU admission. Ninety-three (45.81%) patients needed mechanical ventilation. The baseline variables were comparable among survivors and non-survivors [Table/Fig-1].
Baseline characteristics of patients.
| Baseline variable | Survivors | Non-survivors |
|---|
| Age in months, Mean (SD) | 46 (±44) | 35 (±40) |
| Gender distribution |
| Males | 46 | 59 |
| Females | 51 | 47 |
| Invasive ventilation required | 26 | 67 |
| PELOD-2 scores on Day 1, Mean (SD) | 5.95 (±2.47) | 12.87 (±4.73) |
| Hospital length of stay, days [Median, (IQR)] | 5 (4-7) | 4(3-5) |
The observed mortality was 106 (52.21%). The mean PELOD-2 score among survivors and non-survivors was 5.95 (±2.47) and 12.87 (±4.73), respectively (p<0.001). The cut-off for mortality predicted by the PELOD-2 score was 7.5. PELOD-2 predicted 117(57.63%) non-survivors out of which 96 were actual non-survivors [Table/Fig-2].
Analysis of expected outcome using PELOD-2 score.
| Observed | Total |
|---|
| Non-survivors | Survivors |
|---|
| Non-survivor | 96 | 21 | 117 |
| Survivor | 10 | 76 | 86 |
| Total | 106 | 97 | 203 |
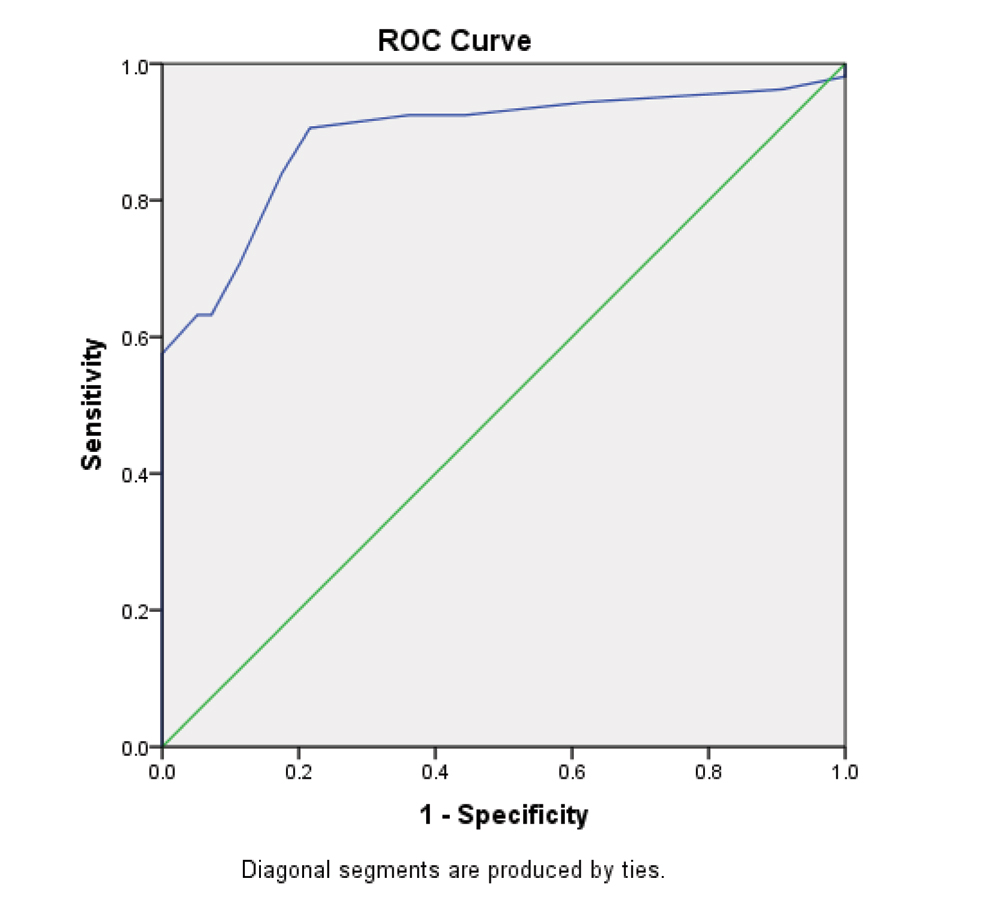
The discriminate analysis of PELOD-2 showed a sensitivity and specificity of 90.56% and 78.35%, respectively [Table/Fig-3].
Screening power of PELOD-2 score.
| Screening power | Values |
|---|
| Sensitivity | 90.56% |
| Specificity | 78.35% |
| AUC | 0.89 (0.84-0.94) |
| SMR | 0.91 (0.74-1.08) |
| Hosmer-Lemeshow test | χ2=2.44 and p-value= 0.11 |
The mortality risk increased with increasing number of organ failures. It increased from 35.7% with 2 organs to 92.68% with 5 organ failures [Table/Fig-4].
Organ dysfunction and PELOD-2 scores.
| Number of Organs Involved | Patients (n) | Mean score (SD) | Mortality (N,%) |
|---|
| 1 | 26 | 3.53 (0.64) | 2 (7.6%) |
| 2 | 28 | 4.85 (1.55) | 10 (35.7%) |
| 3 | 63 | 7.71 (2.35) | 25 (39.68%) |
| 4 | 45 | 13.97 (4.68) | 31 (68.8%) |
| 5 | 41 | 14.63(2.57) | 38 (92.68) |
The SMR of observed and expected mortalities was 0.91(95% CI- 0.74-1.08). The area under ROC curve was 0.89 (0.84-0.94) indicating a good discrimination [Table/Fig-5]. The Hosmer and Lemeshow goodness of fit test showed a good calibration at χ2=2.44 and p-value of 0.11.
ROC curve of PELOD-2 score for mortality.
Discussion
In this study, the performance of PELOD-2 score was evaluated in predicting the outcome in patients of severe sepsis admitted to PICU. The Mean age of children was 37.87 (±46.97) months. In a similar study on performance of PELOD-2 in severe sepsis, the mean age was 22 months [17]. The mean age in the study done by Deshmukh T et al., was 67 months [16].
The median duration of stay at PICU was 4 (3-5) days which is comparable to studies done from different other parts of the world [12,13,18]. The mortality rate (52.21%) was comparable to the mortality rates observed in sepsis and severe sepsis patients [19,20]. The plausible reasons of high sepsis mortality in developing countries include the resource limitation and most of the admissions being done on an emergency rather than on elective basis.
Total 93 (45.81%) patients required mechanical ventilation. The reported rates of mechanical ventilation by Leteurtre S et al., Gonçalves JP et al., Deshmukh T et al., El-Nawawy A et al., Thukral A et al., and Garcia PCR et al., were 52.5%, 68.5%, 17.82%, 52.5%, 25%, and 35.6%, respectively [12,13,16,18,21,22]. The difference in the rates of mechanical ventilation can be attributed to the present study being done in the subset of severe sepsis patients in contrast to all other previous studies done in overall PICU admissions.
In this study, the mortality in 2 OD was 35.7%, which increased to 92.68% in 5 ODs. Deshmukh T et al., reported 1.4% mortality at 0-4 PELOD-2 scores, rising to 66.6% in scores >15 [16]. Thukral A et al., studied PELOD score and found similar rising trends of mortality with rising PELOD scores [21]. El-Nawawy A et al., reported 3.1% mortality in 2 ODs and 80% mortality in 5 ODs [18]. The difference could be explained by the specific subset of severe sepsis patients in this study as opposed to overall PICU admissions studied by El-Nawawy A et al., [18]. Leteurtre S et al., reported 59% of the deceased patients having 5 ODs by PELOD-2 [12]. Hence, the assessment of this score has shown significant relation to the mortality in PICU patients.
The SMR of 0.91 (95% CI- 0.74-1.08) showed overall calibration of the PELOD-2 score as it included the value of 1 in the confidence interval which implied no significant difference between the expected and observed mortality [11,21].
The validation of a score is done by the discrimination, calibration and clinical relevance of the score. The discrimination was done by AUC, where the value of 0.8-0.9 is considered excellent and more than 0.9 is considered outstanding [23]. The AUC discrimination of 0.89 was excellent in this study. It was similar to the AUC found in the studies done by Deshmukh T et al., (AUC 0.87) on PELOD-2 and Thukral A et al., on PELOD (0.8) [16,21]. The AUC was reported as 0.98 in PELOD-2 validation study, and 0.94 and 0.91 reported by Gonçalves JP et al., and El-Nawawy A et al., respectively [13,18].
The calibration was done by Hosmer-Lemeshow chi-square test where a p-value of >0.05 indicates the test to be in good calibration with the population under study, while a value of p-value <0.05 is indicative of poor calibration. The PELOD-2 score showed good calibration with our population at χ2=2.44 and p-value of 0.11. It was reported as p=0.42 by Deshmukh T et al., [16]. The PELOD-2 studies done by Leteurtre S et al., Deshmukh T et al., and El-Nawawy A et al., showed good calibration of the test [12,16,18] while the Portugese study showed poor calibration at 0.02 for which no reason was justified [13].
The clinical relevance of the score lies in its reliable assessment of OD and fairly sensitive and specific prediction of the outcome. The test is objective and the inter-observer differences in the level of assessment of sickness can be avoided. The simplicity of the score with a limited number of variables allow more uniform training of the assessor and consistent reproducibility with different assessors [13,24]. The score correlates the number of organ dysfunction with mortality giving an idea about the utilisation of equipment in a resource limited setup.
The study is possibly the first of its kind reported from India considering the validation of PELOD-2 in paediatric patients of severe sepsis admitted to PICU. The PELOD-2 study by Deshmukh T et al., from South India was done in overall PICU admissions while the present study has been done on subset of “severe sepsis” patients [16]. Leclerc F et al., found Day-1 PELOD-2 SCORES to be highly predictive of mortality among children admitted to PICU with suspected infection [25]. However, more studies form different parts of the world are needed to confirm the validation in and outside of PICU.
Limitation(s)
The patients of severe sepsis admitted outside of PICU were not included in the study. Also, the cases that died within 24 hours of admission were excluded from the study. These children were likely to have arrived in hospital with advanced disease and sometimes in cardio-respiratory failure. Calculation of PELOD-2 scores may not be possible in such scenario.
Conclusion(s)
Day 1 PELOD-2 scores reliably assess the multiple OD and predict outcomes in severe sepsis patients admitted to PICU. The score has comparable validation in the Indian population (developing country) to the original validation study population of France and Belgium (developed countries). It is however reiterated that the main aim of these scores is to describe the multi-organ dysfunction rather than prediction of mortality.