Introduction
LBC has been shown to be more effective than the conventional Pap smear for screening of cervical cancer by significantly improving detection of low-grade and high-grade squamous intraepithelial lesions and a significant improvement in specimen adequacy [1]. The greatest advantage of LBC is that ancillary techniques like HPV testing and DNA ploidy can be performed on the remainder left over sample in the vial. DNA ploidy has been effectively performed for diagnostic and prognostic applications of cervical, ovarian and endometrial cancer. Image cytometry is the most commonly used cytometry technique, others include laser scanning cytometry and flow cytometry. Researchers have utilised liquid based preparation for measurement of DNA content of cervical epithelial cells as it provides satisfactory monolayer for DNA measurement. For flow Cytometric DNA analysis, cells in suspension are required which may be prepared from cervical biopsies or LBC sample. Cell cycle analysis on LBC samples provides useful information for selecting women with chance of developing lesion (Aneuploidy or High S phase fraction) [2-4]. This article provides a review of various Cytometric researches aimed at studying DNA ploidy in cervical cytology samples and to evaluate whether LBC proves to be a suitable sample type for ploidy studies.
Cell Cycle and DNA Ploidy
DNA content of a cell is an essential tool to monitor cell proliferation, cell cycle and DNA ploidy. Cell division undergoes through various phases which form the cell cycle with different amount of DNA content in each phase. Before the cell division starts, the cell remains in a resting phase, known as the G0 phase. As soon as the cell receives signal, the cell starts proliferating and enters G1 phase. In this phase, the cells are diploid and the chromosome number is 2N. The cell then enters S phase which is called the synthesis phase and where the DNA replicates. Replication leads to tetraploidy which contains double the amount of DNA content. This is followed by G2 phase when cell prepares for division and enters the mitosis M phase. The cell in a cell cycle has to overcome two checkpoints G1/S and G2/M. At these checkpoints the cells are checked for DNA damage. These checkpoints prevent the cell to enter into S and M phase, respectively until the damage is repaired. In normal steady state conditions and in low grade/ early lesions, 85% cells are in G0/G1 phase and 15% are in G2/M phase. This anomaly can serve as an efficient diagnostic tool to detect cancer in cells at an early stage [5].
Burden of Cervical Cancer
In women, cancer of the cervix is the 4th most common cancer with 528,000 new cases and 266,000 deaths in 2012. This accounted for 7.5% of all female cancer deaths in 2012. Ninety percent of deaths due to cervical cancer occur in developing regions. The occurrence and mortality due to cervical cancer is highest in Africa and Melanesia [6].
It was estimated in 2015 that every year 122,844 women are diagnosed with cancer of cervix and 67,477 deaths are contributed by cervical cancer in India. It is 2nd most common cancer in females of reproductive age group. In general population, 5% women are expected to harbour HPV-16/18 infection, and most of the invasive cervical cancers (83.1%) are attributed to HPV-16/18 [7].
Cervical Cancer Screening and Diagnosis
Invasive squamous carcinoma of the cervix is the result of pre-invasive lesions known as Cervical Intraepithelial Neoplasia (CIN). In histology CIN is graded as mild dysplasia (CIN 1), moderate dysplasia (CIN 2) and severe dysplasia (CIN 3). Out of these CIN 1 and 2 may regress but CIN 3 progresses to the invasive carcinoma [8,9]. The Bethesda system has improved the reporting and classified it as Negative for intraepithelial lesion or malignancy (NILM), Atypical Squamous Cells of Undetermined Significance (ASC-US), Low grade Squamous Intraepithelial Lesions (LSIL), High Grade SIL (HSIL) and Invasive Carcinoma [10].
Cervical cancer is caused by the HPV infection which induces CIN lesions in the cervix [11-13]. Dysregulated viral oncogene expression caused by integration of viral oncogene in affected cells results in chromosomal instability, aneuploidization and progression of the disease [14]. Epigenetic changes and interference of the viral oncogene in the normal cell cycle may also lead to variation in nuclear DNA content [12,15]. There is evidence that chromosomal instability and aneuploidisation precede and favour high risk HPV genome [14]. Studies have shown that the variation in the ploidy content indicates invasive carcinoma or prospective neoplastic development in cervical dysplasia [16,17]. However, there is no technique which can predict cervical dysplasia clinically with high sensitivity.
Pap test plays important role in screening of cervical carcinoma; however its sensitivity and specificity is limited. It has been reported by Sulik SM et al., (2001) that LBC is more sensitive (90%; 95% CI 77-96%) compared to conventional cytology (79%; 95% CI: 59-91%) for CIN 2 or more severe lesions [18]. LBC has been found to be equivalent or superior to conventional cytology for CIN diagnosis. False positive rate of pre-malignant and malignant lesions by Pap test is approximately 30% and false-negative rate lies between 6-55% [19-23].
Analysis of cervical biopsies has shown that women who develop LSIL have a probability to develop moderate to severe CIN [13]. To diagnose and prevent cervical malignancy a number of diagnostic techniques have been developed. One such technique for the assessment of DNA ploidy to detect cervical dysplasia is DNA cytometry. DNA ploidy has been identified as a prognostic factor for estimation of risk of progression of cervical lesions to invasive cervical carcinoma [24-29]. Aneuploidy aids in identification of dysplasia and provides a predictive value for malignant transformation [30]. Cytometric techniques provide additional information for identification of dysplasia and neoplasia beyond morphology.
Methodology for DNA Ploidy Estimation and Interpretation of Results
DNA image cytometry: Several researchers have utilised the method described below with minor variations to estimate DNA ploidy in LBC. After preparing a second monolayer from the remaining LBC sample, slides are air dried and fixed in buffered formalin for 30 minutes. Following 1-hour acid hydrolysis (5N hydrochloric acid) at room temperature staining with Feulgen (Thionin) is carried out. For calibration of each staining procedure calibration slides are added. Image cytometry is then performed using ploidy measurement software on image cytometer. By and large the interpretation of DNA histogram is similar in all studies with recognition of diploid, polyploid, aneuploid peaks and S Phase fraction. Some researchers have suggested minor modifications in interpretation which are as follows: Auer GU et al., (1980) presented the DNA ploidy value as a “c” for chromosome [31]. The DNA cytometry histogram was classified as normal or suspect; normal corresponding to diploid with low proliferation fraction and polyploid (diploid + tetraploid) histograms without any cells exceeding 5c. All other histograms with any of these were regarded as suspect and patients with suspect results underwent colposcopy.
Any cells with DNA content >5c
Diploid cells with >10% cells in proliferation fraction
Aneuploid cell population
Study of Bollmann M et al., 2006, suggested interpretation of the DNA histogram which is as follows:
Diploid as DNA peak between 1.8c and 2.2c.
Minimum of 2 stem lines with DNA peaks between 1.8c-2.2c and 3.6-4.4c or around 8c and 16c to be read as polypoid.
DNA peaks beyond “diploid” or “Polyploid” peaks and/or presence of single cells with DNA content >9c to be read as aneuploid [32].
Further variation in the histogram interpretation was suggested by Guillaud M et al., 2006 [33]. They defined DNA aneuploidy as a function of three parameters:
Total number of counted cells on a slide;
A DNA ploidy index, beyond which a cell is called aneuploid; and
A cut-off value presenting the number of cells, beyond which a specimen is called aneuploid.
The DNA ploidy index was determined within the range of 2c-9c. The aneuploid cells were determined in the range of 1-50 cells. The sensitivity and specificity was calculated by combining the above definitions to find the best diagnostic accuracy. In a number of studies, 2c DNA content is defined as a diploid cell, 4c as tetraploid cell and 5c as a cut off for aneuploid cell, however Bollmann R et al., Bollmann R et al., and Lorenzeto M et al., suggest 9c [34-36]. Number of cells with DNA exceeding beyond 5c is frequently called the 5c-exceeding rate (5cER) [37].
Flow cytometry for DNA ploidy estimation: Researchers have analysed DNA ploidy by flow cytometry in various solid tumours and LBC samples [38-44]. Single cell suspension was prepared by mechanical or enzymatic disaggregation of the tissue followed by staining with Propidium Iodide (PI) containing Ribonuclease (RNase) for 30-60 minutes at 4°C before analysing on flow cytometer. Gates were set up on FL2W versus FL2A dot plot to exclude doublets and aggregates. FL2A area signals were then used to generate single parameter DNA histograms. Usually two major peaks are observed; one peak is labelled as diploid and another one as an aneuploid (if present). A sample with single G0/G1 peak is defined as diploid, while a sample with two distinct G0/G1 peaks is considered as DNA aneuploid. DNA Index (DI) for aneuploid cells is obtained by dividing the mean channel number of the aneuploid G0/G1 peak by the mean channel number of the diploid G0/G1 peak. For diploid cells DI corresponds to 1 while DI≠1.0 defines aneuploidy. Coefficient of Variation (CV) of G0/G1peak is used to check the quality of DNA histogram. Some studies have also used ModFit software for analysis of DNA histogram.
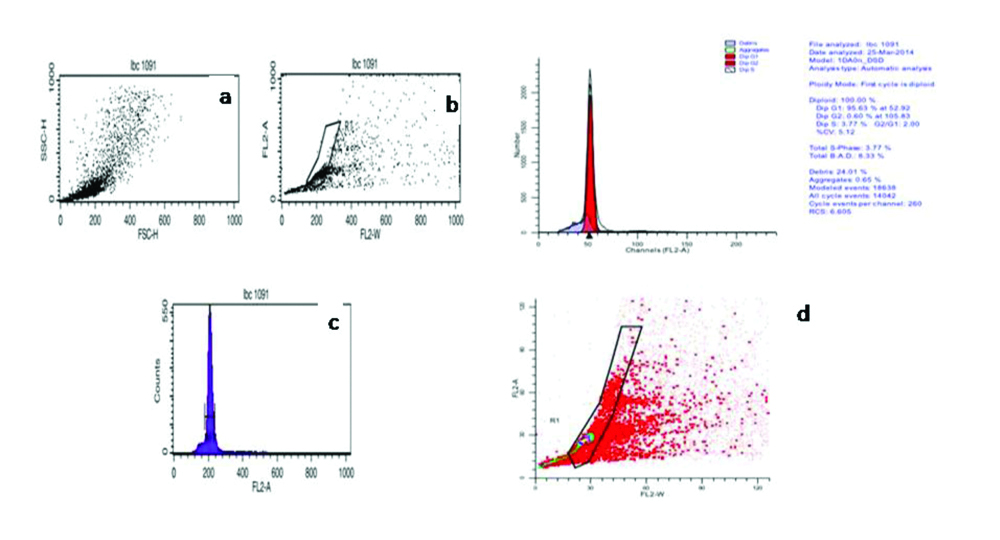
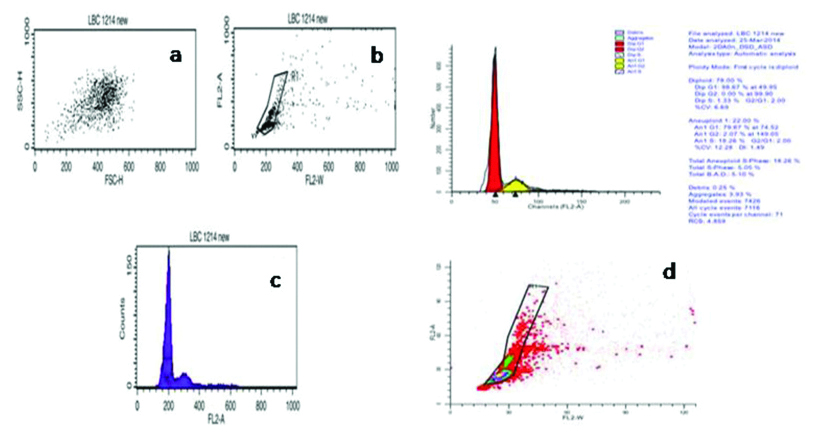
Authors have successfully standardised flow cytometry to assess DNA ploidy in LBC samples of cervical pre cancer and cancer. Cytologically confirmed cases of LSIL, HSIL and SCC along with cases negative for intraepithelial lesion or malignancies (NILM) were used as control for DNA ploidy analysis. Briefly, LBC samples were centrifuged to obtain a cell pellet and washed with equal volume of Phosphate buffer saline (PBS, pH-7.4). Cells were stained with Telford reagent and processed as per Mishra S et al., [45]. Stained cells were acquired using flow cytometer and dot plot and histograms as shown in [Table/Fig-1a,b,2a,b]. Diploid samples were identified by the presence of single G0/G1 peak [Table/Fig-1c,d], while aneuploidy was defined when DI≠1.0 [Table/Fig-2c,d].
Shows the acquisition of cervical epithelial cells on flow cytometer, stained with Telford Reagent. (a-c) Shows acquisition of stained cells on FSC vs. SSC, FL2-A vs. FL2-W and FL2-A vs. Count on Cell Quest Pro software (B.D Biosciences, Singapore). (d) Shows the analysis of acquired FCS file on ModFit LT 3.2 (Verity Software House). Based on ModFit analysis case was found to be Diploid with single G0/G1 peak. Histogram statistics showed on top right.
Image courtesy Mishra et al. [45]
Shows an Aneuploid case of HSIL on cytomorphology acquired on flow cytometer, stained with Telford Reagent. (a-c) Shows acquisition of stained cells on FSC vs. SSC, FL2-A vs. FL2-W and FL2-A vs. Count on Cell Quest Pro software (B.D Biosciences, Singapore). (d) Shows the analysis of acquired FCS file on ModFit LT 3.2 (Verity Software House). Based on ModFit analysis case was found to be Aneuploid on appearance of second G0/G1 population to the right of first G0/G1 peak with DNA index of 1.49.
Image courtesy Mishra et al. [45]
Laser Scanning Cytometry
This technique uses the 2nd monolayer slide stained with Propidium iodide and RNase for 1 hour at 37°C. After the incubation slides were mounted in glycerol and covered with glass. Using the laser scanning cytometer, at least 10,000 cells were measured and diploid and aneuploid cells were defined as per the first peak intensity of DNA histogram containing normal leucocytes. The Coefficients Of Variation (CV) were reported in a range between 4.0 and 7.5. Cells with elevated DNA content as stained by PI (>5c and >9c) were individually evaluated. Haroske G et al., defines, isolated cells with non-superficial cell morphology and a DNA content of greater than 9c as “Rare cells” with abnormally high DNA content [30].
Evaluation of Ploidy as a Diagnostic Procedure in Cervical Cancer
DNA ploidy measurement has been established as a prognostic factor and to be of prognostic significance in ovarian and endometrial cancer though in cervical cancer there are conflicting results [46-52]. In few studies, flow cytometric analysis of DNA ploidy in CIN and invasive cervical has been reported to have prognostic significance for estimation of disease progression into more advanced lesion [38]. The published researches aimed to study the value of DNA ploidy by image cytometry as well as flow cytometry on LBC and solid tissue are summarised in the [Table/Fig-3]. According to most of the studies, HPV typing and DNA ploidy measurement helps in the identification of cytologic dysplasia. LBC has proven to be suitable and useful tool for performing DNA ploidy.
Summary of the studies assessing DNA Ploidy in LBC samples of cervical cytology and solid tissues.
| S. No. | Author (years) | Objective of the study | Number of cases | Result | Conclusion |
|---|
| 1. | Lorenzato M et al. (2002) [36] | To study the usefulness of DNA ploidy measurement on LBC smears showing conflicting results between cytology and HR-HPV typing using Image Cytometry | Total 7944 cases out which 984 underwent ploidy | Normal DNA profile predicted clearance of HPV with sensitivity 81.5%, specificity 45.4%, PPV 69% and NPV 62.4%In persistent HR-HPV infection suspected DNA profile PPV increased from 10.8% to 22.7%, for HSIL detection sensitivity was 95.2% | Cytometry should be complemented with HR-HPV test to select women with a high risk for developing a histologic lesion. |
| 2. | Bollmann R et al. (2003) [35] | To determine HPV typing and DNA ploidy of squamous intraepithelial lesions in LBC samples using Laser Scanning Cytometry | 112 SIL cases | Out of 112 cases, 110 (98.2%) were HPV+, out of these 95 (84.8%) were HR-HPV+ and 46 out of 95 (48.4%) presented aneuploid squamous cells with >9c DNA content. | Complex analysis of cervical lesions from LBC samples is highly informativeHPV typing and DNA ploidy measurement helps in the objectivation of cytologic atypia and both can be performed efficiently from the same LBC sample. |
| 3. | Shirata NK et al. (2003) [55] | To evaluate nuclear DNA content of cervical lesions in LBC specimens using Static Image Cytometry | Total 47 samples out of which CIN1;n=25, CIN2;n=5, CIN3;n=2 and chronic cervicitis=15 | Chronic cervicitis All diploidCIN1 44% diploid, 12% tetraploid, 32% aneuploid, 12% polyploidCIN2 60% diploid, 40% aneuploidCIN3 100% aneuploid | LBC proved to be suitable and highly useful for DNA analysis.Discrimination could be made between CIN3 and CIN1,2 but not between CIN1 and CIN2 |
| 4. | Guillaud M et al. (2006) [33] | To compare DNA ploidy with HPV-testing and conventional cervical cytology as a primary screening test for HSIL and cancer using Image Cytometry | 1555 patients | Cytology Sensitivity 54% Specificity 93% PPV 41% NPV 92%HPV Testing Sensitivity 91% Specificity 80% PPV 70% NPV 90%DNA ploidy Sensitivity 61% Specificity 91% PPV 59% NPV 93% | DNA ploidy shows comparable sensitivity, specificity, PPV and NPV values to conventional cytology and HCIIDNA ploidy is semi-automated and can be performed in less than 8 hours. |
| 5. | Yu XR et al., (2011) [56] | To perform cell quantitative analysis of DNA ploidy in cervical cancer screening using Image Cytometry | 776 women | Conventional Cytology Sensitivity 61.9% Specificity 98.3%DNA ploidy Sensitivity 83.6% Specificity 96.7% | Automated DNA cytometry may be a useful tool for cervical cancer screening in developed countries and has a competitive sensitivity and specificity compared to conventional cytology. |
| 6. | Tong H et al., (2009) [57] | To perform DNA ploidy cytometry testing for cervical cancer screening in China using Image Cytometry | 11,999 women for DNA cytometry testing and 11,994 women for cytologic testing | Diagnosis of cancer:DNA cytometry-40Cytology-24 | DNA cytometry is more beneficial in mass cervical cancer screening with greater sensitivity and positive predicted value than the conventional cytology testing in the developing countries. |
| Cytometry Sensitivity 91.7% Specificity 54.1% ConventionalCytology Sensitivity 44.5% Specificity 70.6%Cytology & Cytometry Sensitivity 100% Specificity 91.8% |
| 7. | Li Z et al., (2010) [58] | To reduce the false-negative rates of population based cervical screening programs employing conventional cytology in combination with automated DNA Image cytometer | 3603 women | Total diagnosis: 51 cases including, 27 CIN2, 16 CIN3 and 8 Invasive cancer cases.Cytology No. of Diagnosis 29 Sensitivity 56.8% Specificity 86.2%DNA Cytometry No. of Diagnosis 38 Sensitivity 74.5% Specificity 81.5%Cytology & Cytometry No. of Diagnosis 42 Sensitivity 82.4% Specificity 81.5% | Screening for high grade neoplastic lesions and cervical cancer by DNA Image cytometer or combination of conventional cytology and DNA Image cytometer is more sensitive than conventional cytology. |
| 8. | Saxena M et al., (2010) [39] | Could addition of DNA content study using flow cytometry improves the detection of cervix cancer | Total of 100 including 38 normal and 62 cancer of cervix cases. | Fraction of Total S phase, TotalAneuploid and G2-M (Diploid) are significantly higher (p < 0.01); while G0-G1 (Diploid)and G0-G1 (Aneuploid) are significantly lower (p <0.01) in cancer patients as compared to control.G0-G1 (Diploid) Sensitivity-96.77% Specificity-100%Total S phase or AneuploidSensitivity-100%Specificity 100% | G0-G1 (Diploid) may help in the diagnosis of carcinoma of the cervix which correlates well with histologically confirmed varied grading of cervical cancer as well as patient survival. |
| 9. | Singh M et al., (2008) [38] | Study the DNA content by flow cytometry and to compare it with the cytological findings. | 184 Cytologicallydiagnosed cases of mild (79),moderate (36), and severe (12) dysplasia along with 57 cases of ASCUS and 69 controls | Aneuploidy was found in 39/79 of mild, 28/36 of moderate, 11/12 of severe dysplasia, 8/57 of ASCUS and in 6/69 controls. | DNA flow cytometry can detect progressive lesions with the greatest possible sensitivity and specificity. |
| 10. | Melsheimer P et al., (2004) [13] | DNA Aneuploidy and Integration of Human Papillomavirus Type 16 E6/E7 Oncogenes in Intraepithelial Neoplasia and Invasive Squamous Cell Carcinoma of the Cervix Uteri | Total 85 samples out of which CIN1/2 n=20, CIN3 n=50,Cacx=15 | DNA aneuploidyCIN1/2= 4/20CIN3= 16/50Cacx= 12/15HPV E6/E7 integrationCIN1/2=1/20CIN3=7/50Cacx=12/15 | Aneuploidization precedes integration of HR-HPV genomes in the progression of cervical dysplasia. |
| 11. | Mishra S et al., (2017) [45] | Flow cytometric Analysis of DNA Ploidy in Liquid Based Cytology of Cervical Pre-cancer and Cancer | 50 Cytologically diagnosed cases of Cervical cancer including 10 LSIL, 20 HSIL, 20 SCC and 31NILM cases as control | Mean diploid G1 values lowered significantly (p<0.0) while diploid S values were significantly (p<0.01) higher in both HSIL and SCC as compared to control | Diploid G1 and diploid S phase analysis do not appear to increase the overall sensitivity and specificity of detection. |
Discussion
DNA ploidy has proved to be an effective tool in detecting high grade neoplastic lesions which helps in the early screening of cancer. Compared to conventional cytology, DNA Cytometry has better sensitivity and specificity. Among the various kinds of cytometry, image cytometry has been widely used and has given positive results in detecting neoplastic lesions. Although flow cytometry is a common modality for studying DNA ploidy in cell suspension viz., blood cells and body fluids, there are only few studies available for assessment of DNA ploidy by Flow Cytometry in LBC samples [38,39].
[Table/Fig-3] suggests that LBC sample is suitable enough to study DNA ploidy and other ancillary techniques. In a Study by Saxena M et al., sensitivity and specificity for diploid G0/G1 to discriminate the cases from controls was 96.77% and 100%, however total S phase and aneuploidy revealed 100% sensitivity [39]. In contrast to this, Singh M et al., reported aneuploidy in 51.31% mild, 77.77% moderate and 91.66% severe cases. In ASCUS, aneuploidy was found in 14.03% cases and interstingly, in 8.69% of controls [38]. Authors further suggested that cases which were found aneuploid should be followed-up for developing advanced grade lesion.
When both cytometry and conventional cytology tests are considered in combination, the figures rise up to 100% and 91.8%, respectively. Though these additional tests improve the sensitivity and specificity, it increases the cost. DNA ploidy analysis appears to be an attractive technology for established programs [33].
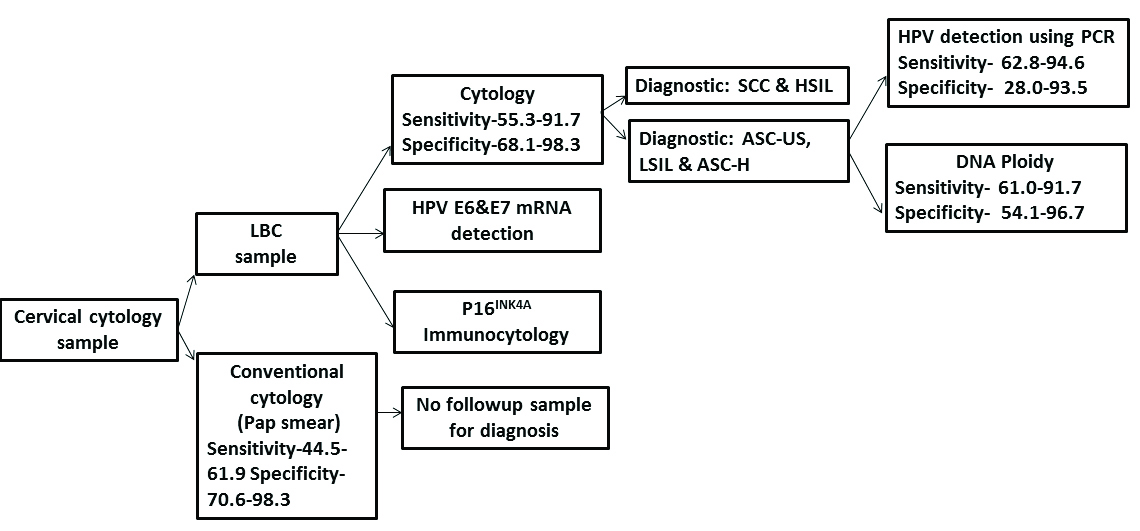
[Table/Fig-4] represents the diagnostic efficacies of various techniques used for the diagnosis of pre cancer/cancer in a cervical sample and suggests a diagnostic algorithm for cervical cancer screening. The LBC is much sensitive and specific as compared to the conventional cytology. Depending on the grade of intraepithelial neoplasia, further workup on HPV testing or DNA ploidy can be carried out. As seen in the [Table/Fig-3] sensitivity of HPV testing is high whereas the specificity of DNA ploidy is high, hence as proposed by various authors DNA cytometry when used in association with HPV testing or conventional cytology gives a better sensitivity and specificity [34-37,53]. Apart from these techniques, some other techniques have also been successfully tried on LBC samples and they are immunocytology using p16INK4a marker and HPV E6/E7 mRNA detection [54]. They can be used along with the other techniques to increase the diagnostic accuracy [55-58].
Suggested diagnostic algorithm for cervical cancer screening.
Conclusion(s)
Cytometry when coupled with HPV DNA typing or the conventional cytology gives better results as compared to that of conventional cytology or DNA cytometry alone. Thus, LBC media provide a good and stable source of cervical cells to carry out ploidy studies using DNA Cytometry. The procedure should be used in conjunction with LBC and HPV detection. Liquid based preparation allows to measure DNA content of cervical epithelial cells that provides more accurate and sensitive result which can alternatively serve as a marker for early stage diagnosis. Use of LBC sample for measurement of DNA content of cervical epithelial cells in the form of aneuploidy or high S phase fraction provides an objective method to prognosticate and select women who may be developing lesions.
[1]. Monsonego J, Autillo-Touati A, Bergeron C, Dachez R, Liaras J, Saurel J, Liquid-based cytology for primary cervical cancer screening: A multi-centre studyBr J Cancer 2001 84:360-66.10.1054/bjoc.2000.158811161401 [Google Scholar] [CrossRef] [PubMed]
[2]. Nghiem VT, Davies KR, Beck JR, Follen M, MacAulay C, Guillaud M, Economic evaluation of DNA ploidy analysis vs liquid-based cytology for cervical screeningBr J Cancer 2015 112(12):1951-57.10.1038/bjc.2015.9525919612 [Google Scholar] [CrossRef] [PubMed]
[3]. Garner D, Clinical application of DNA ploidy to cervical cancer screening: A reviewWorld J Clin Oncol 2014 5(5):93110.5306/wjco.v5.i5.93125493231 [Google Scholar] [CrossRef] [PubMed]
[4]. Sun XR, Wang J, Garner D, Palcic B, Detection of cervical cancer and high grade neoplastic lesions by a combination of liquid-based sampling preparation and DNA measurements using automated image cytometryAnal Cell Pathol 2005 27(1):33-41.10.1155/2005/98161215750205 [Google Scholar] [CrossRef] [PubMed]
[5]. Tay DL, Bhathal PS, Fox RM, Quantitation of G0 and G1 phase cells in primary carcinomas. Antibody to M1 subunit of ribonucleotide reductase shows G1 phase restriction point blockJ Clin Investig 1991 87(2):519-27.10.1172/JCI1150261991836 [Google Scholar] [CrossRef] [PubMed]
[6]. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012Int J Cancer 2012 136:E359-86.10.1002/ijc.2921025220842 [Google Scholar] [CrossRef] [PubMed]
[7]. Mello V, Sundstrom RK, Cervical Intraepithelial Neoplasia (CIN)StatPearls [Internet] 2020 Aug 12 [Google Scholar]
[8]. Khieu M, Butler SL, High grade squamous intraepithelial lesion (HSIL)StatPearls [Internet] 2020 Apr 27 [Google Scholar]
[9]. Omori M, Hashi A, Nakazawa K, Yuminamochi T, Yamane T, Hirata S, Estimation of prognoses for cervical intraepithelial neoplasia 2 by p16INK4a immunoexpression and high-risk HPV in situ hybridization signal typesAm J Clin Pathol 2007 128(2):208-17.10.1309/0UP5PJK9RYF7BPHM17638654 [Google Scholar] [CrossRef] [PubMed]
[10]. Melsheimer P, Klaes Rv, Knebel Doeberitz M, Bastert G, Prospective clinical study comparing DNA flow cytometry and HPV typing as predictive tests for persistence and progression of CIN I/IICytometry A: The Journal of the International Society for Analytical Cytology 2001 46(3):166-71.10.1002/cyto.110111449407 [Google Scholar] [CrossRef] [PubMed]
[11]. Nayar R, Wilbur DC, The Bethesda system for reporting cervical cytology: Definitions, criteria, and explanatory notes 2015 Apr 13 Springer10.1007/978-3-319-11074-5 [Google Scholar] [CrossRef]
[12]. Kashyap V, Das DK, Luthra UK, Micro photometric nuclear DNA analysis in cervical dysplasia of the uterine cervix: Its relation to the progression to malignancy and regression to normalcyNeoplasma 1990 37(5):497-500. [Google Scholar]
[13]. Melsheimer P, Vinokurova S, Wentzensen N, Bastert G, von Knebel Doeberitz M, DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteriClin Cancer Res 2004 10(9):3059-63.10.1158/1078-0432.CCR-03-056515131043 [Google Scholar] [CrossRef] [PubMed]
[14]. Scheurer ME, Guillaud M, Tortolero-Luna G, McAulay C, Follen M, Human papillomavirus-related cellular changes measured by cytometric analysis of DNA ploidy and chromatin textureCytometry B: Clin Cytom 2007 72(5):324-31.10.1002/cyto.b.2017317205571 [Google Scholar] [CrossRef] [PubMed]
[15]. Winkler B, Crum CP, Fujii T, Ferenczy A, Boon M, Braun L, Koilocytotic lesions of the cervix. The relationship of mitotic abnormalities to the presence of papillomavirus antigens and nuclear DNA contentCancer 1994 53(5):1081-87.10.1002/1097-0142(19840301)53:5<1081::AID-CNCR2820530511>3.0.CO;2-L [Google Scholar] [CrossRef]
[16]. Cotton SC, Sharp L, Little J, Duncan I, Alexander L, Cruickshank ME, Trial of management of borderline and other low-grade abnormal smears (TOMBOLA): Trial designContemp Clin Trials 2006 27(5):449-71.10.1016/j.cct.2006.04.00116765101 [Google Scholar] [CrossRef] [PubMed]
[17]. Anton M, Nenutil R, Rejthar A, Kopecny J, Ptackova B, Zaloudik J, DNA flow cytometry: A predictor of a high-risk group in cervical cancerCancer Detect Prev 1997 21(3):242-46. [Google Scholar]
[18]. Sulik SM, Kroeger K, Schultz JK, Brown JL, Becker LA, Grant WD, Are fluid-based cytologies superior to the conventional Papanicolaou test? A systematic reviewJournal of Family Practice 2001 50(12):1040-47. [Google Scholar]
[19]. Richart RM, Vaillant HW, Influence of cell collection techniques upon cytological diagnosisCancer 1965 18(11):1474-78.10.1002/1097-0142(196511)18:11<1474::AID-CNCR2820181117>3.0.CO;2-V [Google Scholar] [CrossRef]
[20]. Coppleson LW, Brown B, Estimation of the screening error rate from the observed detection rates in repeated cervical cytologyAm. J Obst Gynecol 1974 119(7):953-58.10.1016/0002-9378(74)90013-1 [Google Scholar] [CrossRef]
[21]. Gay JD, Donaldson LD, Goellner JR, False-negative results in cervical cytologic studiesActa Cytologica 1985 29(6):1043-46. [Google Scholar]
[22]. Koss LG, The Papanicolaou test for cervical cancer detection: A triumph and a tragedyJAMA 1989 261(5):737-43.10.1001/jama.1989.034200500870462642983 [Google Scholar] [CrossRef] [PubMed]
[23]. Zahniser DJ, Sullivan PJ, Cytyc CorporationActa cytologica 1996 40(1):37-44.10.1159/0003335838604572 [Google Scholar] [CrossRef] [PubMed]
[24]. Bollmann R, Bollmann M, Henson DE, Bodo M, DNA cytometry confirms the utility of the Bethesda system for the classification of Papanicolaou smearsCancer Cytopathol 2001 93(3):222-28.10.1002/cncr.9033 [Google Scholar] [CrossRef]
[25]. Horn LC, Raptis G, Nenning H, DNA cytometric analysis of surgically treated squamous cell cancer of the uterine cervix, stage pT1b1-pT2bAnal. Quant. Cytol. Histol 2002 24(1):23-29. [Google Scholar]
[26]. Böcking A, Motherby H, Assessment of cervical dysplasia with DNA image cytometryDer Pathologe 1999 20(1):25-33.10.1007/s00292005031610091229 [Google Scholar] [CrossRef] [PubMed]
[27]. Duesberg P, Li R, Rasnick D, Aneuploidy approaching a perfect score in predicting and preventing cancer: Highlights from a conference held in Oakland, CA in January, 2004Cell Cycle 2004 3(6):823-28.Available at: https://www.tandfonline.com/doi/pdf/10.4161/cc.3.6.93810.4161/cc.3.6.938 [Google Scholar] [CrossRef]
[28]. Giroud F, Haroske G, Reith A, Böcking A, Part II: Specific recommendations for quality assuranceAnal Cell Pathol 1998 17(4):201-08.10.1155/1998/23765910391372 [Google Scholar] [CrossRef] [PubMed]
[29]. Demirel D, Akyürek N, Ramzy I, Diagnostic and prognostic significance of image cytometric DNA ploidy measurement in cytological samples of cervical squamous intraepithelial lesionsCytopathology 2013 24(2):105-12.10.1111/cyt.1203923331643 [Google Scholar] [CrossRef] [PubMed]
[30]. Haroske G, Baak JP, Danielsen H, Giroud F, Gschwendtner A, Oberholzer M, Fourth updated ESACP consensus report on diagnostic DNA image cytometryAnal Cell Pathol 2001 23(2):89-95.10.1155/2001/65764211904464 [Google Scholar] [CrossRef] [PubMed]
[31]. Auer GU, Caspersson TO, Wallgren AS, DNA content and survival in mammary carcinomaAnal Quant Cytol 1980 2(3):161-65. [Google Scholar]
[32]. Bollmann M, Várnai AD, Griefingholt H, Bánkfalvi A, Callenberg H, Speich N, Predicting treatment outcome in cervical diseases using liquid-based cytology, dynamic HPV genotyping and DNA cytometryAnticancer Res 2006 (2B):1439-46. [Google Scholar]
[33]. Guillaud M, Benedet JL, Cantor SB, Staerkel G, Follen M, MacAulay DNA ploidy compared with human papilloma virus testing (Hybrid Capture II) and conventional cervical cytology as a primary screening test for cervical high-grade lesions and cancer in 1555 patients with biopsy confirmationCancer 2006 107(2):309-18.10.1002/cncr.2199316773634 [Google Scholar] [CrossRef] [PubMed]
[34]. Bollmann R, Méhes G, Speich N, Schmitt C, Bollmann M, Aberrant, highly hyperdiploid cells in human papillomavirus-positive, abnormal cytologic samples are associated with progressive lesions of the uterine cervixCancer Cytopathol 2005 105(2):96-100.10.1002/cncr.2084815662700 [Google Scholar] [CrossRef] [PubMed]
[35]. Bollmann R, Méhes G, Torka R, Speich N, Schmitt C, Bollmann M, Human papillomavirus typing and DNA ploidy determination of squamous intraepithelial lesions in liquid-based cytologic samplesCancer Cytopathol 2003 99(1):57-62.10.1002/cncr.1095312589647 [Google Scholar] [CrossRef] [PubMed]
[36]. Lorenzato M, Bory JP, Cucherousset J, Nou JM, Bouttens D, Thil C, Usefulness of DNA ploidy measurement on liquid-based smears showing conflicting results between cytology and high-risk human papillomavirus typingAm J Clin Pathol 2002 118(5):708-13.10.1309/6NXC-V9XD-YM87-8FAE12428790 [Google Scholar] [CrossRef] [PubMed]
[37]. Böcking A, Nguyen VQ, Diagnostic and prognostic use of DNA image cytometry in cervical squamous intraepithelial lesions and invasive carcinomaCancer Cytopathol 2004 102(1):41-54.10.1002/cncr.1188914968417 [Google Scholar] [CrossRef] [PubMed]
[38]. Singh M, Mehrotra S, Kalra N, Singh U, Shukla Y, Correlation of DNA ploidy with progression of cervical cancerJ. Cancer Epidemiol 2008 (1):110.1155/2008/29849520445775 [Google Scholar] [CrossRef] [PubMed]
[39]. Saxena M, Negi MP, Singh S, Singh PK, Singh U, Bhatt ML, DNA content can improve the detection and prognosis of carcinoma of the cervixBiosci Trends 2010 4(3) [Google Scholar]
[40]. Munteanu D, Zlei M, Ailiesei O, Chifu C, Diaconu C, Carasevici E, Flowcytometric evidence of DNA ploidy in human breast cancerJ Prev Med 2006 12:59-65. [Google Scholar]
[41]. Cufer T, Lamovec J, Bracko M, Lindtner J, Us-Krasovec M, Prognostic value of DNA ploidy in breast cancer stage I-IINeoplasma 1997 44:127-32. [Google Scholar]
[42]. Blanco R, Rengifo CE, Cedeño M, Frómeta M, Rengifo E, Flow cytometric measurement of aneuploid DNA content correlates with high S-phase fraction and poor prognosis in patients with non-small-cell lung cancerInternational Scholarly Research Notices 2013 :2013L35412310.1155/2013/354123 [Google Scholar] [CrossRef]
[43]. Tripathi P, Tripathi AK, Kumar A, Ahmad R, DNA aneuploidy study for early detection of chromosomal abnormality in patients with aplastic anemia: Prognostic and therapeutic implicationsIn vivo 2008 22(6):837-44. [Google Scholar]
[44]. Nunez R, DNA measurement and cell cycle analysis by flow cytometryCurr Issues Mol Biol 2001 3:67-70. [Google Scholar]
[45]. Mishra S, Awasthi NP, Husain N, Anand A, Pradeep Y, Ansari R, Flow Cytometric Analysis of DNA Ploidy in Liquid Based Cytology for Cervical Pre-Cancer and CancerAsian Pac J Cancer Prev 2017 18(6):1595-1601.Available at: https://pubmed.ncbi.nlm.nih.gov/28669173/ [Google Scholar]
[46]. Vergote IB, Kærn J, Abeler VM, Pettersen EO, De Vos LN, Tropé CG, Analysis of prognostic factors in stage I epithelial ovarian carcinoma: Importance of degree of differentiation and deoxyribonucleic acid ploidy in predicting relapseAm J of Obstet Gynecol 1993 169(1):40-52.10.1016/0002-9378(93)90129-7 [Google Scholar] [CrossRef]
[47]. Kaern J, Tropé CG, Kristensen GB, Tveit KM, Pettersen EO, Evaluation of deoxyribonucleic acid ploidy and S-phase fraction as prognostic parameters in advanced epithelial ovarian carcinoma: A prospective studyAm J of Obst Gynecol 1994 170(2):479-87.10.1016/S0002-9378(94)70215-2 [Google Scholar] [CrossRef]
[48]. Erba E, Ubezio P, Pepe S, Vaghi M, Marsoni S, Torri W, Flow cytometric analysis of DNA content in human ovarian cancersBr J Cancer 1989 60(1):45-50.10.1038/bjc.1989.2172803914 [Google Scholar] [CrossRef] [PubMed]
[49]. Evans MP, Podratz KC, Endometrial neoplasia: Prognostic significance of ploidy statusClinical obstetrics and gynecology 1996 39(3):696-706.10.1097/00003081-199609000-000178862893 [Google Scholar] [CrossRef] [PubMed]
[50]. Podratz KC, Wilson TO, Gaffey TA, Cha SS, Katzmann JA, Deoxyribonucleic acid analysis facilitates the pre-treatment identification of high-risk endometrial cancer patientsAm J O Gynecol 1993 168(4):1206-13.10.1016/0002-9378(93)90370-X [Google Scholar] [CrossRef]
[51]. Willen R, Himmelmann A, Långström-Einarsson E, Fernö M, Ranstam J, Baldetorp B, Prospective malignancy grading, flow cytometry DNA-measurements and adjuvant chemotherapy for invasive squamous cell carcinoma of the uterine cervixAnticancer Res 1993 13(4):1187-96. [Google Scholar]
[52]. Jakobsen A, Ploidy level and short-time prognosis of early cervix cancerRadiother Oncol 1984 1(3):271-75.10.1016/S0167-8140(84)80010-9 [Google Scholar] [CrossRef]
[53]. Abulafia O, Pezzullo JC, Sherer DM, Performance of ThinPrep liquid-based cervical cytology in comparison with conventionally prepared Papanicolaou smears: A quantitative surveyGynecol Oncol 2003 90(1):137-44.10.1016/S0090-8258(03)00176-8 [Google Scholar] [CrossRef]
[54]. Reuschenbach M, Clad A, von Knebel Doeberitz C, Wentzensen N, Rahmsdorf J, Schaffrath F, Griesser H, Freudenberg N, von Knebel Doeberitz M, Performance of p16INK4a-cytology, HPV mRNA, and HPV DNA testing to identify high grade cervical dysplasia in women with abnormal screening resultsGynecol Oncol 2010 119(1):98-105.10.1016/j.ygyno.2010.06.01120619445 [Google Scholar] [CrossRef] [PubMed]
[55]. Shirata NK, Longatto FA, Roteli-Martins C, Espoladore LM, Pittoli JE, Syrjänen K, Applicability of liquid-based cytology to the assessment of DNA content in cervical lesions using static cytometryAnal Quant Cytol Histol 2003 25(4):210-14. [Google Scholar]
[56]. Yu XR, Liu Y, Wang X, Kuang Ql, Li Xf, Kuang Cy, Cell quantitative analysis of DNA ploidy in cervical cancer screeningChinese Journal of Diagnostic Pathology 2011 4:019 [Google Scholar]
[57]. Tong H, Shen R, Wang Z, Kan Y, Wang Y, Li F, Wang F, Yang J, Guo X, Mass Cervical Cancer Screening Regimen GroupDNA ploidy cytometry testing for cervical cancer screening in China (DNACIC Trial): A prospective randomized, controlled trialClin. Cancer Res 2009 15(20):6438-45.10.1158/1078-0432.CCR-09-168919825960 [Google Scholar] [CrossRef] [PubMed]
[58]. Li Z, Zhang M, Li H, Improved Detection of Cervical Cancer and High Grade Neoplastic Lesions by a Combination of Conventional Cytology and DNA Automated Image CytometerJ. Cancer Ther 2010 1(02):4710.4236/jct.2010.12008 [Google Scholar] [CrossRef]