Introduction
Osteoporosis is a affliction characterised by decreased bone mass and mineral density with poor bone quality predisposing to fracture in both men and women and is the most common bone disease [1]. The presence of fragility fractures predominates the clinical features of severe osteoporosis. Osteoporosis and the resulting fragility fractures are major public health burdens both humanly and economically. Prevention remains the priority as once fractures occur; associated morbidity also becomes a concern. Due to the absence of warning signs before a fracture, patients usually do not receive effective therapy during the initial phase of osteoporosis to prevent fracture morbidity [2].
The classification of osteoporosis into primary and secondary forms is whimsical. Secondary Osteoporosis is described as an underlying disorder, deficiency, or medication causing the bone fragility or reduced bone mass, whereas osteoporosis in women with natural menopause and older men with is called primary osteoporosis [1]. Incidence of secondary causes of osteoporosis has been reported to be ranging from 30%-60% in men, more than 50% in pre-menopausal women, and about 30% of post-menopausal [3]. Secondary osteoporosis remains a challenge for the endocrinologist as it usually affects younger men and women where a diagnosis of osteoporosis is not usually suspected. The diagnostic challenges also include the myriad of underlying disorders that are diverse and rare and require specific diagnostic tests [4,5]. [Table/Fig-1] enumerates the causes of secondary osteoporosis. Diabetes mellitus- both type 1 and 2, Acromegaly, Growth Hormone (GH) deficiency, Hyperparathyroidism, Hyperthyroidism, hypercortisolism including Cushing syndrome, Hypogonadism both primary and secondary, hyperprolactinemia, Pregnancy, lactation, and hyperaldosteronism are the endocrinopathies associated secondary osteoporosis.
Causes of secondary osteoporosis.
| Underlying disorders | Causes |
|---|
| Endocrinopathies | Diabetes mellitus (both type 1 and 2), Acromegaly, GH deficiency, Hyperparathyroidism, Hyperthyroidism, hypercortisolism including Cushing syndrome, Hypogonadism -both primary and secondary, hyperprolactinemia, Pregnancy and lactation, hyperaldosteronism |
| Congenital/genetic conditions | a) Structural collagen and connective tissue defects: Osteogenesis imperfecta, pseudoxanthoma elasticum, Ehlers-Danlos syndrome, Marfan syndrome, Menkes syndrome. b) Disrupted phosphate and/or calcium homeostasis: idiopathic hypercalciuria, hypophosphatasia. c) Metabolic/ storage disorders: Gaucher disease, cystic fibrosis, glycogen storage diseases, haemochromatosis, porphyria
|
| Autoimmune disorders | Rheumatoid Arthritis (RA), Lupus erythematosus, Multiple sclerosis, Ankylosing spondylitis |
| Neoplastic and Hematologic disorders | Leukaemia; haemophilia, sickle cell anaemia, thalassaemia, metastatic disease; myelofibrosis, lymphoma; multiple myeloma; systemic mastocytosis; monoclonal Gammopathy of unknown significance. Breast and prostate cancer |
| Gastrointestinal Disorders | Coeliac disease, Weight loss surgery, Gastrectomy and other causes of malabsorption including chronic pancreatitis; liver cirrhosis; Inflammatory bowel disease, chronic biliary tract obstruction and alcohol related malnutrition |
| Nutritional deficiency and disorders | Deficiency of minerals such as calcium, magnesium, protein and/or vitamin D; parenteral nutrition; scurvy, anorexia nervosa, bulimia nervosa, malnutrition |
| Neurologic and psychiatric disorders | Stroke, Parkinson disease, multiple sclerosis, depression, eating disorders, Spinal cord injuries, post-polio syndrome |
| Drugs | Acid suppression therapies: omeprazole, pantoprazole etc.Anti-coagulants: warfarin, heparinAnti-convulsants: valproate; phenytoin; carbamazepineAnti-depressants: selective serotonin reuptake inhibitorsAnti-hormonal therapies: aromatase inhibitorsAnti-manic therapies: lithiumAnti-psychotic therapiesAnti-retroviral drugs: tenofovirContraceptives: progesteroneCytotoxic drugs (chemotherapy): cyclosporine, tacrolimus, methotrexateDiuretics: furosemideGlucocorticoids Gonadotrophin-releasing hormone analogs: buserelin; goserelin; cyproterone acetateLipase inhibitors: orlistatThiazolidinediones: rosiglitazone; pioglitazoneThyroid hormone: L-thyroxineUnfractionated heparins: dalteparin; enoxaparin; tinzaparin |
| Infective | HIV/AIDS, poliomyelitis |
| Chronic illness | Liver disease, kidney disease, COPD |
| Others | Organ transplant, weight loss, female athletic triad |
HIV: Human immunopdeficiency virus; AIDS: Acquired immunodeficiency syndrome; COPD: Chronic obstructive pulmonary syndrome
Hence, the present study aims to summarise the current knowledge regarding the endocrine causes of secondary osteoporosis.
Mechanisms
Glucocorticoid-Induced Osteoporosis (GIO)
The most pertinent cause of secondary osteoporosis is glucocorticoid-induced osteoporosis, which is usually results from the use of various steroids for treatment of inflammatory or autoimmune disorders [6]. Glucocorticoids are frequently given to patients with inflammatory and autoimmune disorders, and these primary diseases themselves are frequently associated with bone loss and osteoporosis. Systemic release of inflammatory cytokines has significant effects on bone remodeling and is associated with bone loss in Rheumatoid Arthritis (RA), Inflammatory Bowel Disease (IBD) and Systemic Lupus Erythematosus (SLE). Glucocorticoids increase, due to whether endogenously over-production or administered systemically, produces profound skeletal effects. The usual presentation of GIO is often a fragility fracture, occurring in 30-50% of patients [7]. Doses >7.5 mg/day lead to a 5 fold increased risk and even low-dose prednisolone (2.5-7.5 mg/day) correlates with a 2.6-fold increase in vertebral fractures [8]. There is significant bone improvishment and escalated marrow adipogenesis as marrow stromal cells transform to the fat lineage on prolonged exposure to pharmacologic doses of glucocorticoids. Both the osteoclast and osteoblast are affected directly, and secondary hypogonadism, secondary hyperparathyroidism, impaired vitamin D metabolism, muscle atrophy, and hypercalciuria occur during glucocorticoid therapy. All of these aspects lead to an expeditious and prolonged bone loss during the initial duration of steroid therapy [9,10].
[Table/Fig-2] illustrates the direct effect of glucorticoids on bone. Glucocorticoids effects on the bone formation are mediated mainly through FGF-21 mediated increased expression of Peroxisome Proliferator-Activated Receptor-Gamma Receptor (PPARγ) [11] and the Wnt/β-catenin signaling pathway [12,13]. The PPARγ mechanism causes the divergence of pluripotent precursor cells to adipocytes in preference to osteoblasts, causing a decrease in the numbers of osteoblasts. Glucocorticoids also directly affect the bone resorption, escalating the production of macrophage colony-stimulating factor (M-CSF) and Receptor activator of nuclear factor kappa-B ligand (RANKL) and reducing the production of Osteoprotegerin (OPG) by osteoblastic cells and osteocytes, increasing both in the number and activity of osteoclasts.
Direct effects of glucocorticoids on bone.
FGF 21: Fibroblast growth factor 21; OPG: Osteoprotegenrin; RANKL: Receptor activator of nuclear factor kappa-B ligand; PPARγ: Peroxisome proliferator-activated receptor-gamma receptor; M-CSF: Macrophage colony-stimulating factor
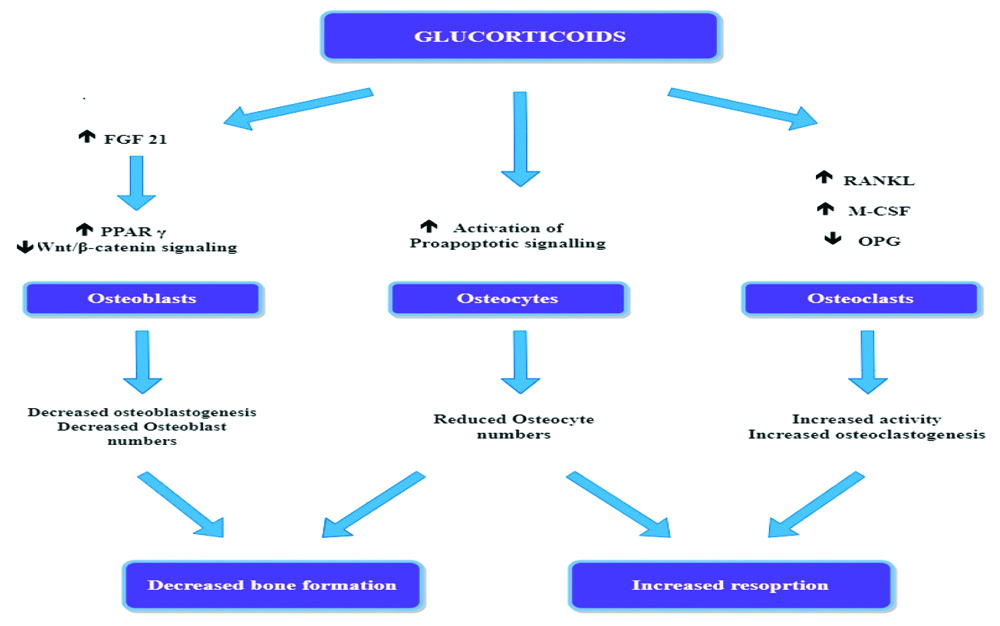
The variation in bone effect severity may be due to pre-receptor changes in glucocorticoid activity by 11 beta-hydroxysteroid dehydrogenase enzyme and polymorphisms in the glucocorticoid receptor [14,15]. Glucocorticoids have a strong adverse effect on bone formation by curbing the expression of Insulin-Like Growth Factor-1 (IGF1) in bone cells and by causing the transformation of marrow stromal cells into the fat lineage rather than into the osteoblast differentiation pathway [16]. Other factors that may be additive to GIO include hypogonadism, diminished physical activity due to myopathy, increased renal and intestinal losses of calcium, impaired production of growth hormone, and IGF1 Binding Protein (IGF-BP) [17]. Glucocorticoid excess also has negative effects on muscle mass and function, leading to myopathy and subsequent risk of falls [18].
Trabecular Bone Score (TBS) is a measure of trabecular bone architecture which is obtained from dual-energy X-ray absorptiometry (DXA) of the L1-L4 lumbar spine and can predict fracture independent of BMD [19]. In a study of 64 postmenopausal women taking prednisolone in a doses of ≥5 mg daily for >3 months, TBS was markedly reduced than the controls, although there was not much difference in the lumbar spine BMD T-scores [20]. A study on 416 patients on long-term predisolone (≥5 mg daily for 3 months) reported similar findings, with the reduction in TBS being most marked in men and those with a fracture [21]. These findings may provide evidence that steroids adversely affects the spine bone microarchitecture and these effects are independent of Bone Mineral density and may be a factor in the increased fracture risk.
GIO occurs at an indistinguishable frequency in men and women and various ethnic groups but is more incessant in elderly patients and in those with underlying inflammatory disorders, low BMI, and/or pre-existing impaired bone density [7]. The risk of fractures at all sites was nevertheless rapidly reversed after stopping glucocorticoids. The accruing dose may be less important than the daily dose [22].
Hyperthyroidism, Thyroid Hormone Replacement, and Suppressive Therapy
The clinical impact of thyrotoxicosis on bone health was first demonstrated in the 19th century as ‘worm-eaten’ long bones found postmortem in patients deceased due to primary thyrotoxicosis. Hyperthyroidism reduces the bone remodeling cycle duration increasing the resorption and causing increased bone turnover state with more bone resorption and formation rates. There is an elevation in the frequency of initiation of bone remodeling. Bone formation and mineralisation are reduced to a greater extent than bone resorption. This leads to decreased bone mineralization, a net 10% decrease in bone in each remodeling cycle, causing osteoporosis [23]. Depressed serum TSH levels and hyperthyroidism are correlated with an increased risk of hip and vertebral fractures with additive effects of increased falls due to decreasing muscle strength and coordination [24-26]. Besides, any current therapy with thyroid hormone replacement is inversely associated with BMD and propogates the probability of fractures even in the presence of euthyroidism [27].
Thyrotoxicosis causes increased elimination of calcium and phosphorus in both urine and stool; with subsequent elevation in bone turnover. Presence of vitamin D deficiency [28] and myopathy in graves may predispose to increased chances of osteoporosis. Thyroid hormone (T3) accelerates the activity of the osteoclasts via its nuclear receptors and this effect may explain these widespread changes. Local actions of TSH which may normally balance thyroid hormone action on osteoclasts and enhance osteoblast activity may also have a role. This effect is absent in hyperthyroidism [25,29-32].
Primary Hyperparathyroidism
Primary Hyperparathyroidism (PHPT) leads to an elevated expression of RANKL by the osteoblast lineage and an elevation in osteoclast-mediated bone resorption. Although there is an increase in osteoblast activity and associated bone formation, it is not enough to supplant the enhanced bone resorption. Bone turnover markers are in usual range or mildly elevated. In PHPT the cortical bone is mostly involved while the trabecular bone is preserved comparatively. Excess parathyroid hormone results in cortical thinning due to endosteal bone resorption while there is no effect on trabecular bone, so that BMD in the distal forearm and the hip are reduced more [33,34].
Diabetes
While both Type 1 (T1DM) and Type 2 diabetes (T2DM) puts the patient at an elevated risk of fragility fractures, the risk is more with type I than with type II DM and peculiar differences exist between bone disorders in T1DM and T2DM [35,36]. There is bone loss in T1DM and it is recognised as a risk factor for osteoporosis and bone fractures. In T2DM, bone mass is preserved or increased providing evidence that the bone quality instead of quantity is the main causative reason affecting bone strength in this disorder. Also, the drugs used in the treatment of diabetes may cause bone loss and osteoporosis.
The peculiar difference in the secretion of growth factors and adipokines between T1DM and T2DM certainly is another point to be taken into consideration. T1DM is usually associated with low serum levels of IGF1, whereas this is not part of the hormonal alterations in T2DM. T2DM also predominantly exhibits an inflammatory profile in adipokine secretion by the white adipose tissue that is represented by increased levels of leptin, chemerin, resistin, Tumour Necrosis Factor (TNF), and Interleukins (IL), whereas adiponectin is reduced. Leptin and adiponectin have many complex effects on bone, and there are still no conclusive results about their ultimate effects on bone [37].
Thiazolidinediones are exogenous agonists of PPARs that lead to bone marrow mesenchymal stromal cells differentiation into adipocytes, inhibiting osteoblastogenesis via decreasing the Runx2 transcription factor, as well as IGF1 and Wnt signaling pathways. Thiazolidinediones also lead to osteoclast differentiation and bone resorption, and patients may experience bone loss and low BMD leading to an increased risk of fractures [38-42].
In older patients with a prolonged duration of T1DM and poor diabetic control, because there are increased vascular complications which may lead to reduced bone mass and increased fracture risk. Men with T1DM seem to be especially susceptible to osteopenia or osteoporosis [43]. Among patients with recent-onset T1DM, the impaired bone formation has been hypothesized to be due to the bone anabolic effects of insulin and amylin [44].
Type 2 DM patients are at an elevated risk of fracture with even normal or increased BMD [45]. This apparent conundrum may be interpreted by decreased bone quality in patients with T2DM, as a consequence of hyperinsulinemia, deposition of end-products from advanced glycosylation, renal failure, hypercalciuria, microangiopathy, and/or reduced serum levels of IGF-1. Fracture risk in T2DM might be increased by visual complications, muscle wasting, neuropathy, and impaired balance, leading to a greater risk of falls [43].
Hypogonadism
Hypogonadism, whether primary, secondary or drug-induced is associated with decreased BMD and osteoporosis, hypogonadism is the primary physiological cause of osteoporosis. Conditions such as premature menopause and drugs, such as aromatase inhibitors and gonadotropin hormone-releasing hormone (GnRH) analogs which cause hypogonadism, lead to reduced BMD, and increase the risk of fractures. While estrogen deficiency is major factor in the development of osteoporosis in both men and women, it is more of a factor causing postmenopausal osteoporosis.
Osteoblasts have androgen receptors, and testosterone and 5α- DHT (5α- dihydrotestosterone) both stimulate osteoblast to differentiate [46]. Testosterone may also increase skeletal and circulating IGF1 and stimulate bone formation, thus its decrease may be one of the additional causes that leads to bone loss in hypogonadal men [47].
Testosterone and 5α-DHT regulate gene expression in osteoblasts leading to inhibition of the resorptive capacity of isolated osteoclasts. In addition, sex hormones affect the release of a various cytokines and growth factors including Macrophage Colony Stimulating Factor (MCSF), and the proinflammatory molecules Interleukin-1 (IL-1), Interleukin-6 (IL-6) and TNF-α, RANKL, and OPG, which modulate the effects of androgens on bone remodeling [48-51].
Growth Hormone Deficiency (GHD) and Acromegaly
GH increases bone formation directly via action on the GH receptors in osteoblasts and via local production of IGF1. GHD leads to delayed skeletal maturation and reduction in BMD amongst patients with isolated GHD and in patients with multiple pituitary deficiencies, mainly through reduced bone formation [52].
Acromegaly is associated with elevated bone remodelling, when the skeleton is exposed to an increased levels of IGF-I for long duration, changes in bone microstructure may occur with a consequent reduction in bone strength and patients with acromegaly have a markedly higher prevalence of vertebral fractures, which is associated with the duration of the disease and serum IGF-1 levels. The hypercalciuria has been noticed in acromegaly patients and is traditionally linked to enhanced intestinal calcium absorption driven by calcitriol, as well as to the elevated bone turnover induced by GH excess and thus may be considered a marker of skeletal fragility [53,54].
Pregnancy and Lactation
Changes in bone mass in association with both pregnancy and lactation have been reported in several studies. At the lumbar spine, longitudinal studies show losses of 3-5% over pregnancy and 3-10% over six months of lactation with the recovery of the bone mass demonstrated over 6-12 months, thereafter, even in the setting of continued lactation. Over six months of lactation, a bone loss of 2-4% has been documented at the hip. The amount of bone loss during lactation is directly proportional to longer durations of lactation and postpartum amenorrhea [55-59].
There are several hypotheses regarding the cause of osteoporosis of pregnancy and lactation. These include the release of PTHrp (parathyroid related peptide) [60], increased transfer of calcium from bone to central circulation to provide calcium needed for lactation and fetal bone [61] formations, pre-existing osteopenia before pregnancy, decreased oestrogen after pregnancy leading to increased activity of osteoclasts, and stimulate bone resorption through OPG-RANK-RANKL pathway [62,63], the lordotic posture of pregnancy and the increased weight-bearing might induce thoracic or lumbar fractures [64] and inactivity during pregnancy from bed rest or hospitalisation would increase bone loss [65], however the exact aetiopathogenesis still eludes us [21].
Hyperprolactinemia
Hypogonadism is believed to be the major mechanism by which these patients develop low BMD, due to abnormalities in the normal pulsatile secretion of GnRH [66]. A study showed there is an presence of prolactin receptors on osteoblasts, and prolactin treated osteoblasts had a reduced proliferation with an overall elevated rate of apoptosis. There was also reduced mineralization as suggested by decreased calcium content in these cells [67].
Evaluation
History and physical examination
To elucidate the risk factors for fractures, the underlying disease, and potential drugs, a detailed history alongwith physical examination should be performed with special attention to the following factors.
The medical history should include information on:
Adult and childhood fractures
Adult and childhood illnesses and medication exposures
Menstrual history
Timing of recent pregnancy or lactation
Dieting and exercise behaviour
Gastrointestinal symptoms
Nephrolithiasis
Family history of osteoporosis and/or nephrolithiasis
Evaluation of nutritional status, calcium and vitamin D intake
An exhaustive review of all medications partaken is necessary, as is assement of the smoking and alcohol habits, and the hereditary disposition of osteoporosis or fractures.
Physical examination should be performed to looks for signs of: [Table/Fig-3]:
Clinical clues to endocrine causes of adult secondary osteoporosis.
| Endocrine disorder | Clinical features |
|---|
| Cushing’s syndrome | Weight gain, moon facies, buffalo hump, Pink or purple broad striae on the skin of the abdomen, thighs, breasts, and arms, proximal muscle weakness, thin fragile skin that bruises with minor trauma |
| Primary hyperparathyroidism | weakness, polydipsia, polyuria, nocturia, joint pain, bone pain, nephrolithiasis, nausea, vomiting, pancreatitis |
| Hyperthyroidism | Unintentional weight loss, tachycardia, palpitations, nervousness, anxiety, irritability, tremors, excessive sweating, sensitivity to heat, goiter |
| Hypogonadism | Underdeveloped secondary sexual characters and genitals, Disproportionate growth of the limbs in relation to the trunk of the body (eunochoidal habitus), hot flashes, and vasomotor symptoms in females, fatigue, and lethargy. |
| Diabetes | Polydipsia, polyuria unexplained weight loss, fatigue, delayed wound healing, frequent infections, Type 2 diabetes mellitus individuals can be obese and have signs of insulin resistance |
| Growth hormone deficiency | Decreased muscle mass and strength, Difficulty to concentration, Fatigue and/or tiredness, history of pituitary surgery or irradiation |
| Acromegaly | Enlarged hands and feet, Coarse enlarged facial features, Coarse oily thickened skin, Excessive sweating |
Nutritional deficiency or eating disorder
Cushing syndrome
Thyroid hormone excess
Connective tissue disorders and structural collagen disorders (e.g. Osteogenesis imperfecta, Ehlers Danlos syndrome, Marfan syndrome),
Inflammatory and Rheumatological conditions conditions (e.g. rheumatoid arthritis, SLE)
Once detailed history and examination have been performed the next step is to perform the relevant laboratory evaluation.
Laboratory and radiological evaluation: Initial and specific laboratory and radiological evaluations are summarised in [Table/Fig-4].
Laboratory and radiological evaluation of secondary osteoporosis.
| Investigations | Diagnostic implication |
|---|
| Complete blood count, peripheral blood film | May show anaemia |
| Renal and liver function test | Deranged function may be a feature of primary disease (diabetic nephropathy, Hyperparathyroidism) or may contribute to osteoporosis itself |
| Serum calcium and phosphate levels | Levels reflect underlying disease states (severe hypercalcemiacaemia and low phosphorus may reflect hyperparathyroidism) |
| Serum bone-specific or total ALP activity | Elevated levels in hyperparathyroidism |
| Serum 25-hydroxyvitamin D | Inadequate vitamin D levels predispose to osteoporosis |
| 24-h urinary calcium excretion, protein, phosphorus (with creatinine control) | Assesses for hypercalciuria (e.g., hyperparathyroidism, cushings disease, hyperthyroidism) |
| Bone turnover markers | Formation markers indicate high turnover states (Elevated in patients with hyperparathyroidism, acromegaly, cushings and reduced in hypothyroidism, GHD) |
| X-ray lumbar and thoracic spine with a skeletal survey | To assess overall skeletal integrity, diagnosis of fractures, specific signs such as sub-periosteal bone resorption, acro-osteolysis, brown tumours etc., |
| DEXA (Bone mineral density measurement) | Gold standard for diagnosing osteoporosis |
| Quantitative Computed Tomography (QCT) | It estimates BMD as a true volume density in g/cm3, which is not affected by bone size. Most sensitive method of diagnosing osteoporosis, not widely available |
| Quantitative Ultrasound (QUS) | Heel is the only validated skeletal site for the clinical use of QUS, advantage of not using radiation, low cost portable, lacks precision |
| Diagnostic conditions | Specific investigations |
| Cushing’s disease | Serum (8 am), ACTH, Morning fasting serum cortisol after dexamethasone suppression, CT abdomen, MRI brain |
| Hyperparathyroidism | Intact parathyroid hormone, USG and CECT neck, Tc99 Sestamibi Scan |
| Hyperthyroidism | T3, T4, TSH, USG neck |
| Hypogonadism | Serum testosterone levels in men, LH, FSH |
| Diabetes | Fasting glucose levels, HbA1c |
| Growth hormone deficiency | IGF-1, GH stimulation test, MRI sella |
| Acromegaly | IGF-1, GH suppression test, MRI sella |
| Hyperprolactinemia | Prolactin |
ALP: Alanine phosphatase; h: hour, GHD: Growth hormone deficiency; DEXA: Dual energy x-ray absorptiometry; BMD: Bone mineral density; ACTH: AdrenoCortical thyrotropin hormone; CT: Computed tomography; MRI: Magnetic resonance imaging; USG: Ultrasonography; CECT: Contrast-enhanced computed tomography; Tc99: Technetium 99; T3: Triiodothyronin, T4: Tetraiodothyronin, TSH: Thyroid stimulating hormone; LH: Leutinizing hormone; FSH: Follicle stimulating hormone; IGF: Insulin-like growth factor
Diagnosis
Diagnosis of secondary osteoporosis requires a high index of suspicion in the younger population and is usually made in the setting of a low trauma fracture i.e., trauma equivalent to a fall from a standing height or less. DXA is used to make a diagnosis of osteoporosis. The International Society for Clinical Densitometry (ISCD) recommendation is to use BMD Z scores rather than T scores at the lumbar spine, hip, and forearm. A Z score ≤ −2.0 indicates “below the expected range for age” and a Z score > −2.0 indicates “within the expected range for age [68]. For postmenopausal t score of < −2.5 is considered as osteoporosis [69].
Management
Once identified, the essential concept of treatment of secondary osteoporosis is to manage the underlying cause wherever possible including surgery drug discontinuation, and switching over to other therapy. If drug discontinuation is not possible, then the lowest possible dose should be used and the patient should be assessed for osteoporotic therapy.
Treatment of the predisposing disease is the best course of action with the increase in BMD over time and a reduction in the possibility of fracture. Intervention in the form of medical therapy would include vitamin D treatment in deficient patients, change of medication, testosterone treatment in hypogonadal men, decreasing the dose of corticosteroids, if possible, in GIO, and correction of metabolic disorders or malabsorption [70,71].
Ensuring ample consumption of calcium (800-1200 mg/day) via dietary uptake or supplements is recommended in all patients with risk of osteoporosis or on treatment with drugs that can cause osteoporosis. Vitamin D replacement (at least 800 IU/day) is recommended as vitamin D deficiency has a high prevalence and may contribute to reduced bone mass and increase the propensity to falls, in addition to various adverse extraskeletal effects [72]. Use of bisphosphonates, teriparatide, Denosumab, and Selective Estrogen Receptor Modulators (SERM) is advised in high-risk patients irrespectively of the cause and especially cause of secondary osteoporosis cannot be treated. The guideline is available for the management of GIO and is discussed below.
Glucocorticoid induced osteoporosis: Removal of ACTH secreting pituitary tumor or cortisol secreting adrenal tumour forms the primary basis for the management of Glucorticoid induced osteoporosis. In the case of exogenous causes of Cushing’s disease stopping glucocorticoid therapy should be considered and if not possible then medical management of osteoporosis should be considered [Table/Fig-5].
Specific management of secondary causes of osteoporosis.
| Cause of osteoporosis | Management |
|---|
| Hyperparathyroidism | Parathyroidectomy results in improvement in BMD. If not a candidate for surgery give bisphosphonates |
| Hyperthyroidism | Maintain TSH in the median range of normal using anti-thyroid medication such as carbimazole and methimazole, surgery and radioiodine ablation may be considered |
| Hypogonadism | Testosterone replacement in males, Oestrogen replacement in females, Selective Oestrogen Receptor Modulators such as Raloxifene. Resumption of normal menstrual function and weight gain appears necessary for skeletal recovery in patients with functional hypothalamic amenorrhea due to anorexia nervosa |
| Acromegaly | Resection of tumours, somatostatin receptor ligands |
| Hyperprolactinemia | Cabergoline, Resection of tumours |
| Growth hormone deficiency | Replacement with recombinant growth hormone |
BMD: Bone mineral density; TSH: Thyroid stimulating hormone
Therapies investigated include bisphosphonates, teriparatide, Selective Estrogen Receptor Modulators (SERMs), and Denosumab of which bisphosphonates (Oral alendronate, and risedronate, and intravenous zoledronic acid) and teriparatide were FDA approved but recently Denosumab has also been approved [Table/Fig-5]. Recent American college of rheumatology guidelines [73] recommends that risk stratification based on BMD, history of fracture, and the use of FRAX (for adults ≥40 years) should be assessed in individuals taking glucocorticoid treatment for a long term, within six months of initiation and repeated with 1-3 yrs; the use of the smallest possible dose of glucocorticoids is recommended to reduce fracture risk. Optimisation of calcium supplementation (1,000-1,200 mg/day) and vitamin D supplementation (600-800 IU/day) and lifestyle adjustments such as a balanced diet, maintaining weight in the recommended range, smoking cessation, regular weight-bearing or resistance training exercise, limiting alcohol intake (1-2 alcoholic beverages/day) is also needed. Various studies have shown the beneficial effects of bisphosphonates on the lumbar spine and hip BMD in people treated with glucocorticoids [74,75] with some showing evidence of a reduction in the rate of vertebral fractures. In a trial comparing the effects of teriparatide and bisphosphonates in GIO, teriparatide showed larger improvements in spinal BMD and TBS. Treatment is indicated in all patients at moderate to high risk and oral bisphosphonate are preferred over IV bisphosphonates, teriparatide, and Denosumab with Raloxifene for post-menopausal women when other therapies cannot be given in the same order of preference. Consideration for changing treatment includes a fracture after >18 months of therapy or > 10 % BMD loss in a year [Table/Fig-4] [74,75].
Denosumab with its action on RANKL provides a novel therapy for GIO, given six monthly ensures better compliance than oral bisphosphonates and a recent randomised double-blind, double-dummy, active-controlled study on the treatment with Denosumab versus risedronate in GIOP showed higher BMD at lumbar spine on comparison to risedronate in both glucocorticoid-continuing (4.4% vs 2.3%) and glucocorticoid-initiating (3.8% vs 0.8%) groups, however rebound increase in bone loss following discontinuation of therapy remains a concern [10].
Osteoporosis Management in diabetes: Glycemic control and lifestyle measures should be adopted to improve general heath in patients with diabetes. Drugs associated with increased risk of fractures i.e., thiazolidinediones and SGLT-2 inhibitors should be avoided in patient at high risk of fractures. Maintainance of a healthy lifestyle with calcium and vitamin D supplementation as needed and exercise to improve muscle strength and reduce the risk of falls also decrease the chances of fractures. In a recent systematic review of nine studies (predominantly trials evaluating alendronate, risedronate, raloxifene, and teriparatide for the treatment of osteoporosis), there were indistinguishable increases in bone density and reductions in vertebral (alendronate, raloxifene) or nonvertebral (teriparatide) fracture risk in patients with and without diabetes [Table/Fig-5] [16].
Conclusion(s)
The strength of this review is that Authors have summated and brought together what is known regarding secondary osteoporosis and identify the gaps in this knowledge providing an avenue for both readers who are new to the topic and well versed with it, to understand and make an informed decision on the topic. The limitations of a literature review of this nature are the excessive dependency on previously available and published studies and while a detailed discussion of management would have been preferable, only an overview could be provided. As the understanding of bone biology and mechanisms of osteoporosis increases, newer pathways and cell signaling systems are discovered and more therapeutic options become available for successful management strategies. In any individual presenting with a recent diagnosis of osteoporosis, screening for the most common secondary causes must be done especially in men and pre-menopausal women. A team consisting of orthopaedicians, rheumatologists, physicians, nurses, physical and occupational therapists can help by early identification of patients, and making appropriate referrals which can improve the number of individuals who receive treatment with medications and thus, improve the osteoporosis and fracture outcomes. A significant number of patients who do not receive such treatment because of affordability and are lost to follow-up, suffer the burden of fractures which can be prevented by understanding the risk factors and providing appropriate management at initial diagnosis.
Author contributions: Sharma H and Sharma B were instrumental to ideation, drafting, writing, and revision of the manuscript. Kumar A and Purwar N performed the literature search and also contributed drafting of the manuscript. Mathur SK and Saran S contributed to the ideation and revising of the manuscript. All authors have revised and accepted the final version of the manuscript.
HIV: Human immunopdeficiency virus; AIDS: Acquired immunodeficiency syndrome; COPD: Chronic obstructive pulmonary syndrome
ALP: Alanine phosphatase; h: hour, GHD: Growth hormone deficiency; DEXA: Dual energy x-ray absorptiometry; BMD: Bone mineral density; ACTH: AdrenoCortical thyrotropin hormone; CT: Computed tomography; MRI: Magnetic resonance imaging; USG: Ultrasonography; CECT: Contrast-enhanced computed tomography; Tc99: Technetium 99; T3: Triiodothyronin, T4: Tetraiodothyronin, TSH: Thyroid stimulating hormone; LH: Leutinizing hormone; FSH: Follicle stimulating hormone; IGF: Insulin-like growth factor
BMD: Bone mineral density; TSH: Thyroid stimulating hormone
[1]. Francisco JA, Dennis MB, Rosen CJ, Osteoporosis Basic and Clinical Aspects. In: Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ editorsWilliams Textbook of Endocrinology 2019 14th edPhiladelphia, USAElsevier Health Sciences:1256-85. [Google Scholar]
[2]. Sözen T, Özışık L, Başaran NÇ, An overview and management of osteoporosisEur J Rheumatol 2017 4(1):46-56.10.5152/eurjrheum.2016.04828293453 [Google Scholar] [CrossRef] [PubMed]
[3]. Hudec SM, Camacho PM, Secondary causes of osteoporosisEndocr Pract 2013 19(1):120-28.10.4158/EP12059.RA23186949 [Google Scholar] [CrossRef] [PubMed]
[4]. Czerwiński E, Czubak J, Synder M, Warzecha M, Berwecka M, Contemporary Management of Osteoporotic FracturesOrtop Traumatol Rehabil 2018 20(2):91-102.10.5604/01.3001.0011.766530152776 [Google Scholar] [CrossRef] [PubMed]
[5]. DiIorgi N, Maruca K, Patti G, Mora S, Update on bone density measurements and their interpretation in children and adolescentsBest Pract Res Clin Endocrinol Metab 2018 32(4):477-98.10.1016/j.beem.2018.06.00230086870 [Google Scholar] [CrossRef] [PubMed]
[6]. Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, A UK Consensus Group on management of glucocorticoid-induced osteoporosis: An updateJ Intern Med 1998 244(4):271-92.10.1046/j.1365-2796.1998.00408.x9797491 [Google Scholar] [CrossRef] [PubMed]
[7]. Kanis JA, johansoon H, Oden A, Johnell O, de Laet C, Melton LJ, A meta-analysis of prior corticosteroid use and fracture riskJ. Bone Miner Res 2004 19(6):893-99.10.1359/JBMR.04013415125788 [Google Scholar] [CrossRef] [PubMed]
[8]. Weinstein RS, Glucocorticoid-induced bone diseaseNEJM 2011 365:62-70.10.1056/NEJMcp101292621732837 [Google Scholar] [CrossRef] [PubMed]
[9]. Rosen CJ, Bouxsein ML, Mechanisms of disease: Is osteoporosis the obesity of bone?Nat Clin Pract Rheumatol 2006 2(1):35-43.10.1038/ncprheum007016932650 [Google Scholar] [CrossRef] [PubMed]
[10]. Canalis E, Mazziotti G, Giustina A, Bilezikian JP, Glucocorticoid-induced osteoporosis: Pathophysiology and therapyOsteoporos Int 2007 18(10):1319-28.10.1007/s00198-007-0394-017566815 [Google Scholar] [CrossRef] [PubMed]
[11]. Guan Y, Huang J, Huang S, Kasukurthi MV, Huang Y, Li D, FGF21 mediates corticosteroid-related bone mass loss through PPAR-gamma2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA 2019 :1915-19.10.1109/BIBM47256.2019.8983042 [Google Scholar] [CrossRef]
[12]. Komori T, Glucocorticoid Signaling and Bone BiologyHorm Metab Res 2016 48(11):755-63.10.1055/s-0042-11057127871116 [Google Scholar] [CrossRef] [PubMed]
[13]. Meszaros K, Patocs A, Glucocorticoids influencing Wnt/β-Catenin pathway; multiple sites, heterogeneous effectsMolecules 2020 25(7):e148910.3390/molecules2507148932218328 [Google Scholar] [CrossRef] [PubMed]
[14]. Swanson C, Lorentzon M, Conaway HH, Lerner UH, Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bonesEndocrinology 2006 147(7):3613-3622.10.1210/en.2005-071716614077 [Google Scholar] [CrossRef] [PubMed]
[15]. Compston J, Glucocorticoid-induced osteoporosis: An updateEndocrine 2018 61(1):07-16.10.1007/s12020-018-1588-229691807 [Google Scholar] [CrossRef] [PubMed]
[16]. Mirza F, Canalis E, Management of endocrine disease: Secondary osteoporosis: pathophysiology and managementEur J Endocrinol 2015 173(3):131-51.10.1530/EJE-15-011825971649 [Google Scholar] [CrossRef] [PubMed]
[17]. Mazziotti G, Formenti AM, Adler RA, Bilezikian JP, Grossman A, Sbardella E, Glucocorticoid-induced osteoporosis: Pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelinesEndocrine 2016 54(3):603-11.10.1007/s12020-016-1146-827766553 [Google Scholar] [CrossRef] [PubMed]
[18]. Sato AY, Richardson D, Cregor M, Davis HM, Au ED, McAndrews K, Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligasesEndocrinology 2017 158(3):664-77. [Google Scholar]
[19]. McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAXJ Bone Miner Res 2016 31(5):940-48.10.1002/jbmr.273426498132 [Google Scholar] [CrossRef] [PubMed]
[20]. Paggiosi MA, Peel NF, Eastell R, The impact of glucocorticoid therapy on trabecular bone score in older womenOsteoporos Int 2015 26(6):1773-80.10.1007/s00198-015-3078-125743176 [Google Scholar] [CrossRef] [PubMed]
[21]. Saag KG, Agnusdei D, Hans D, Kohlmeier LA, Krohn KD, Leib ES, Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatideArthritis Rheumatol 2016 68(9):2122-28.10.1002/art.3972627111239 [Google Scholar] [CrossRef] [PubMed]
[22]. Amiche MA, Abtahi S, Driessen JHM, Vestergaard P, de Vries F, Cadarette SM, Impact of cumulative exposure to high-dose oral glucocorticoids on fracture risk in Denmark: A population-based case-control studyArch Osteoporos 2018 13(1):3010.1007/s11657-018-0424-x29552730 [Google Scholar] [CrossRef] [PubMed]
[23]. Bassett JHD, Williams GR, Role of thyroid hormones in skeletal development and bone maintenanceEndocrine Reviews 2016 37(2):135-87.10.1210/er.2015-110626862888 [Google Scholar] [CrossRef] [PubMed]
[24]. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, Da Costa BR, Subclinical thyroid dysfunction and fracture risk: A meta-analysisJAMA 2015 313(20):2055-65.10.1001/jama.2015.516126010634 [Google Scholar] [CrossRef] [PubMed]
[25]. Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Brix TH, Hegedus L, Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohortJ Bone Miner Res 2014 29(9):2040-50.10.1002/jbmr.224424723381 [Google Scholar] [CrossRef] [PubMed]
[26]. Segna D, Bauer DC, Feller M, Schnieder C, Fink HA, Aurbert CE, Association between subclinical thyroid dysfunction and change in bone mineral density in prospective cohortsJ Intern Med 2018 283(1):56-72.10.1111/joim.1268829034571 [Google Scholar] [CrossRef] [PubMed]
[27]. Vestergaard P, Mosekilde L, Fractures in patients with hyperthyroidism and hypothyroidism: A nationwide follow-up study in 16,249 patientsThyroid 2002 12(5):411-19.10.1089/10507250276004350312097203 [Google Scholar] [CrossRef] [PubMed]
[28]. Dhanwal DK, Kochupillai N, Gupta N, Cooper C, Dennison EM, Hypovitaminosis D and bone mineral metabolism and bone density in hyperthyroidismJ Clin Densitom 2010 13(4):462-66.10.1016/j.jocd.2010.05.00820663698 [Google Scholar] [CrossRef] [PubMed]
[29]. Williams GR, Bassett JHD, Thyroid diseases and bone healthJ Endocrinol Invest 2018 41(1):99-109.10.1007/s40618-017-0753-428853052 [Google Scholar] [CrossRef] [PubMed]
[30]. Zaidi M, Davies TF, Zallone A, Blair HC, Iqbal J, Moonga SS, Thyroid-stimulating hormone, thyroid hormones, and bone lossCurr Osteoporos Rep 2009 7(2):47-52.10.1007/s11914-009-0009-019631028 [Google Scholar] [CrossRef] [PubMed]
[31]. Zaidi M, Yuen T, Sun Li, Rosen CJ, Regulation of Skeletal HomeostasisEndocrine Reviews 2018 39(5):701-18.10.1210/er.2018-0005029897433 [Google Scholar] [CrossRef] [PubMed]
[32]. Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signalingJ Clin Invest 2012 122(10):3737-41.10.1172/JCI6394822996689 [Google Scholar] [CrossRef] [PubMed]
[33]. Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bone disease in primary hyperparathyroidismArq Bras Endocrinol Metabol 2014 58(5):553-61.10.1590/0004-273000000338125166047 [Google Scholar] [CrossRef] [PubMed]
[34]. Makras P, Anastasilakis AD, Bone disease in primary hyperparathyroidismMetabolism 2017 80:57-65.10.1016/j.metabol.2017.10.00329051042 [Google Scholar] [CrossRef] [PubMed]
[35]. Janghorbani M, Feskanich D, Willett WC, Hu F, Prospective study of diabetes and risk of hip fracture: The Nurses’ Health StudyDiabetes Care 2006 29(7):1573-78.10.2337/dc06-044016801581 [Google Scholar] [CrossRef] [PubMed]
[36]. Nicodemus KK, Folsom AR, Iowa Women’s Health S. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal womenDiabetes Care 2001 24(7):1192-97.10.2337/diacare.24.7.119211423501 [Google Scholar] [CrossRef] [PubMed]
[37]. De Paula FJA, Rosen CJ, Structure and function of bone marrow adipocytesComp Physiol 2017 8(1):315-49.10.1002/cphy.c17001029357131 [Google Scholar] [CrossRef] [PubMed]
[38]. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL, Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formationEndocrinology 2005 146(3):1226-35.10.1210/en.2004-073515591153 [Google Scholar] [CrossRef] [PubMed]
[39]. Billington EO, Grey A, Bolland MJ, The effect of thiazolidinediones on bone mineral density and bone turnover: Systematic review and meta-analysisDiabetologia 2015 58(10):2238-46.10.1007/s00125-015-3660-226109213 [Google Scholar] [CrossRef] [PubMed]
[40]. Wan Y, Chong LW, Evans RM, PPAR-gamma regulates osteoclastogenesis in miceNat Med 2007 13(12):1496-503.10.1038/nm167218059282 [Google Scholar] [CrossRef] [PubMed]
[41]. Kheniser KG, Polanco Santos CM, Kashyap SR, The effects of diabetes therapy on bone: A clinical perspectiveJ Diabetes Complications 2018 32(7):713-19.10.1016/j.jdiacomp.2018.04.00529747995 [Google Scholar] [CrossRef] [PubMed]
[42]. Guja C, Guja L, Miulescu RD, Effect of type 2 diabetes medications on fracture riskAnn Transl Med 2019 7(20):58010.21037/atm.2019.09.5131807561 [Google Scholar] [CrossRef] [PubMed]
[43]. Hofbauer L C, Brucck C, Singh SK, Dobnig H, Osteoporosis in patients with diabetes mellitusJ Bone Miner Res 2007 22(9):1317-28.10.1359/jbmr.07051017501667 [Google Scholar] [CrossRef] [PubMed]
[44]. Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowkles JL, Is insulin an anabolic agent in bone? Dissecting the diabetic bone for cluesAm J Physiol Endocrinol Metab 2005 289(5):E735-45.10.1152/ajpendo.00159.200516215165 [Google Scholar] [CrossRef] [PubMed]
[45]. Sellmeyer DE, Civitelli R, Hofbauer LC, Khosla S, Lecka-Czernik B, Schwartz AV, Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetesDiabetes 2016 65(7):1757-66.10.2337/db16-006327329951 [Google Scholar] [CrossRef] [PubMed]
[46]. Manolagas SC, OBrien CA, Almeida M, The role of estrogen and androgen receptors in bone health and diseaseNat Rev Endocrinol 2013 9(12):699-712.10.1038/nrendo.2013.17924042328 [Google Scholar] [CrossRef] [PubMed]
[47]. Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C, Androgens and boneEndocr Rev 2004 25(3):389-425.10.1210/er.2003-000315180950 [Google Scholar] [CrossRef] [PubMed]
[48]. Sfeir J, Drake MT, The Effects of Androgens on Bone Metabolism: Clinical Aspects. In: Leder B, Wein M, editorsOsteoporosis Contemporary Endocrinology 2020 3rd edHumana press:259-75.10.1007/978-3-319-69287-6_13 [Google Scholar] [CrossRef]
[49]. Pederson L, Kremer M, Judd J, Pascoe D, Spelsberg TC, Riggs BL, Androgens regulate bone resorption activity of isolated osteoclasts in vitroProc Natl Acad Sci USA 1999 96(5):505-10.10.1073/pnas.96.2.5059892663 [Google Scholar] [CrossRef] [PubMed]
[50]. Khosla S, Monroe DG, Regulation of bone metabolism by sex steroidsCold Spring Harb Perspect Med 2018 8(1):a03121110.1101/cshperspect.a03121128710257 [Google Scholar] [CrossRef] [PubMed]
[51]. Huber DM, Bendixen AC, Pathrose P, Srivastava S, Dienger KM, Shevde NK, Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factorEndocrinology 2001 142(9):3800-08.10.1210/endo.142.9.840211517156 [Google Scholar] [CrossRef] [PubMed]
[52]. Tritos NA, Klibanski A, Effects of growth hormone on boneProg Mol Biol Transl Sci 2016 138:193-211.10.1016/bs.pmbts.2015.10.00826940392 [Google Scholar] [CrossRef] [PubMed]
[53]. Mazziotti G, Frara S, Giustina A, Pituitary diseases and boneEndocrine Rev 2018 39(4):440-88.10.1210/er.2018-0000529684108 [Google Scholar] [CrossRef] [PubMed]
[54]. Mazziotti G, Biagioli E, Maffezzoni F, Spinello M, Serra V, Maroldi R, Bone turnover, bone mineral density, and fracture risk in acromegaly: A meta-analysisJ Clin Endocrinol Metab 2015 100(2):384-94.10.1210/jc.2014-293725365312 [Google Scholar] [CrossRef] [PubMed]
[55]. Karlsson MK, Ahlborg HG, Karlsson C, Maternity and bone mineral densityActa Orthop 2005 76(1):02-13.10.1080/0001647051003027415788303 [Google Scholar] [CrossRef] [PubMed]
[56]. Karlsson C, Obrant KJ, Karlsson M, Pregnancy and lactation confer reversible bone loss in humansOsteoporos Int 2001 12(10):828-34.10.1007/s00198017003311716185 [Google Scholar] [CrossRef] [PubMed]
[57]. Miyamoto T, Miyakosh K, Sato Y, Kasuga Y, Ikenoue S, Miyamoto K, Changes in bone metabolic profile associated with pregnancy or lactationSci Rep 2019 9:678710.1038/s41598-019-43049-131086225 [Google Scholar] [CrossRef] [PubMed]
[58]. Kolthoff N, Eiken P, Kristensen B, Nielsen SP, Bone mineral changes during pregnancy and lactation: a longitudinal cohort studyClin Sci (Lond) 1998 94(4):405-12.10.1042/cs09404059640346 [Google Scholar] [CrossRef] [PubMed]
[59]. Kovacs CS, Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recoveryPhysiological reviews 2016 96:449-547.10.1152/physrev.00027.201526887676 [Google Scholar] [CrossRef] [PubMed]
[60]. Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosisArthritis Care Res 2017 69(8):1095-110.10.1002/acr.2327928585410 [Google Scholar] [CrossRef] [PubMed]
[61]. Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study GroupNEJM 1998 339(5):292-99.10.1056/NEJM1998073033905029682041 [Google Scholar] [CrossRef] [PubMed]
[62]. Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: A randomised trial. European Corticosteroid-Induced Osteoporosis Treatment StudyJ. Bone Miner. Res 2000 15(6):1006-13.10.1359/jbmr.2000.15.6.100610841169 [Google Scholar] [CrossRef] [PubMed]
[63]. Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, HORIZON investigators. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trialLancet 2009 373(9671):1253-63.10.1016/S0140-6736(09)60250-6 [Google Scholar] [CrossRef]
[64]. Reid DM, Adami S, Devogelaer JP, Chines AA, Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapyCalcif. Tissue Int 2001 69(4):242-47.10.1007/s00223-001-1060-811730260 [Google Scholar] [CrossRef] [PubMed]
[65]. Glüer CC, Marin F, Ringe JD, Hawkins F, Möricke R, Papaioannu N, Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trialJ Bone Miner Res 2013 28(6):1355-68.10.1002/jbmr.187023322362 [Google Scholar] [CrossRef] [PubMed]
[66]. Colangelo L, Biamonte F, Pepe J, Cipriani C, Minisola S, Understanding and managing secondary osteoporosisExpert Rev Endocrinol Metab 2019 14(2):111-22.10.1080/17446651.2019.157572730735441 [Google Scholar] [CrossRef] [PubMed]
[67]. Hsu E, Nanes M, Advances in treatment of glucocorticoid-induced osteoporosisCurr Opin Endocrinol Diabetes Obes 2017 24(6):411-17.10.1097/MED.000000000000036828857847 [Google Scholar] [CrossRef] [PubMed]
[68]. Adami G, Saag K, Glucocorticoid-induced osteoporosis: 2019 concise clinical reviewOsteoporos Int 2019 30(6):1145-56.10.1007/s00198-019-04906-x30805679 [Google Scholar] [CrossRef] [PubMed]
[69]. Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, Denosumab versus risedronate in glucocorticoid-induced osteoporosis: A multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority studyLancet Diabetes Endocrinol 2018 6:445-54.10.1016/S2213-8587(18)30075-5 [Google Scholar] [CrossRef]
[70]. Iwamoto N, Okamoto M, Tsuji S, Endo Y, Takatani A, Shimizu T, Denosumab is effective toward glucocorticoid-induced osteoporosis patients complicated with rheumatic diseases regardless of prior anti-osteoporotic drugsJ Bone Miner Metab 2019 37(3):554-62.10.1007/s00774-018-0955-730187273 [Google Scholar] [CrossRef] [PubMed]
[71]. Yanbeiy ZA, Hansen KE, Denosumab in the treatment of glucocorticoid-induced osteoporosis: A systematic review and meta-analysisDrug Des Devel Ther 2019 13:2843-52.10.2147/DDDT.S14865431616133 [Google Scholar] [CrossRef] [PubMed]
[72]. Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O, Clinical features of 24 patients with rebound associated vertebral fractures after denosumab discontinuation: Systematic review and additional casesJ Bone Miner Res 2017 32(6):1291-96.10.1002/jbmr.311028240371 [Google Scholar] [CrossRef] [PubMed]
[73]. Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Discontinuation of denosumab therapy for osteoporosis: A systematic review and position statement by ECTSBone 2017 105:11-17.10.1016/j.bone.2017.08.00328789921 [Google Scholar] [CrossRef] [PubMed]
[74]. Anagnostis P, Paschou SA, Gkekas NN, Artzouchaltzi AM, Christou K, Stogiannou D, Efficacy of anti-osteoporotic medications in patients with type 1 and 2 diabetes mellitus: A systematic reviewEndocrine 2018 60(3):373-83.10.1007/s12020-018-1548-x29411304 [Google Scholar] [CrossRef] [PubMed]
[75]. Marcocci C, Bollerslev J, Khan AA, Shoback DM, Medical management of primary hyperparathyroidism: proceedings of the fourth International Workshop on the Management of Asymptomatic Primary HyperparathyroidismJ Clin Endocrinol Metab 2014 99(10):3607-18.10.1210/jc.2014-141725162668 [Google Scholar] [CrossRef] [PubMed]