Tonsillectomy, though a short duration and commonly performed surgery in children, pose a great challenge to the anaesthesiologist as well as the surgeon [1]. Extubation in these children is complicated and is associated with a higher risk of laryngospasm due to airway sharing, presence of recurrent respiratory infection and increased incidence of obstructive sleep apnoea. Moreover, there is an increased susceptibility to respiratory events like laryngospasm in the paediatric age group. Consequences of improperly planned extubation include risk of postoperative airway obstruction, aspiration and hypoventilation, all potentially requiring reintubation. Though various techniques and drugs have been tried to decrease the cardiovascular and airway responses during extubation, none proved to be completely successful. A calm and sedated, but arousable child is advantageous after tonsillectomy as they are likely to have lesser risk of postoperative bleeding, which is a much worried complication of this procedure.
Dexmedetomidine, a second generation α2-adrenergic receptor agonist provides sedation, pain relief and anxiolysis without respiratory depression. Dexmedetomidine stimulates central α2 and imidazoline receptors, causes perioperative sympatholysis and decreases blood pressure. This increased α2 selectivity results in sedation, good analgesia and fewer side effects. Dexmedetomidine has effect on endogenous sleep-promoting pathways and the sedation hence resembles natural nonrapid eye movement sleep [2-4].
Dexmedetomidine has evolved as a promising drug for various applications with multiple delivery routes [5,6]. In a recent meta-analysis, Tung A et al., concluded that dexmedetomidine is the most effective drug in decreasing the incidence of moderate to severe coughing during extubation [7]. Studies conducted by Di M et al., and Rani P et al., have cited the beneficial effects of dexmedetomidine in attenuating stress response, providing haemodynamic stability, good emergence and extubation conditions [1,8]. Over the past few years there have been multiple published studies exploring the use of dexmedetomidine in paediatric population [9-11]. It has been found to be beneficial in paediatric anaesthesia due to its anxiolytic, sedative and analgesic properties without significant respiratory depression [11,12].
Fentanyl is a phenylpiperidine-derivative synthetic opioid agonist which is structurally related to meperidine. This phenylpiperidine-derivative is a strong agonist at mu (μ)- and kappa (κ)- opiate receptors, now classified as OP2 (κ) and OP3 (μ) [13]. Fentanyl is an extensively studied and well proven drug to attenuate the respiratory and haemodynamic disturbances of intubation and extubation [14,15].
Even though comparison of dexmedetomidine and fentanyl has been studied in various surgical settings in adults and children, not enough research works are done about effects on extubation in paediatric population. One of the studies by Erdil F et al., observed that intravenous fentanyl 2.5 μg/kg and dexmedetomidine 0.5 μg/kg had similar emergence characteristics, with shorter time to extubation in the dexmedetomidine group [16]. Both groups had similar haemodynamic effects too.
The present study aimed to compare the effects of premedication with single bolus administration of dexmedetomidine 0.5 μg/kg and fentanyl 2 μg/kg intravenously on extubation characteristics in children undergoing tonsillectomy. Primary objective of the study was to compare the extubation quality as well as the time to extubation and eye opening in both groups. Comparison of extubation haemodynamics in both groups and the incidence of any complications like bradycardia, hypotension, excessive sedation, nausea or vomiting in either group were the secondary objectives. The hypothesis of the present study was that dexmedetomidine might provide better extubation characteristics with stable haemodynamics and no delay in recovery compared to fentanyl.
Materials and Methods
After getting Institutional Research and Ethics Committee (IREC) clearance (GMCKKD/ RP 2017/ IEC/243 dated 04/12/2017), this prospective cohort study was conducted in a Tertiary Care Teaching Institute, Government Medical College, Kozhikode, Kerala, India. The study was carried out between December 2017 and September 2019.
Inclusion and Exclusion criteria: Children aged between 5 to 12 years belonging to American Society of Anaesthesiologists’ (ASA) physical status I & II, who were posted for elective tonsillectomy under general anaesthesia were included in this study. Children with active respiratory tract infection, obstructive sleep apnoea symptoms, heart disease or heart block, known drug allergies or bleeding disorders were excluded from the study.
Sample Size Calculation
Based on previous study by Erdil F et al., with an average standard deviation of 3.8, the sample size was calculated to obtain a power of 80% and confidence interval of 95% [16]. To detect a difference of 2 minutes between the groups with an alpha error of 0.05, the calculated sample size was 58 in each group. Assuming some dropouts, 60 children were enrolled in both groups.
Written informed consent was obtained from parents and children were divided into two groups (60 each) according to computer generated random number chart. Group D received dexmedetomidine 0.5 μg/kg and Group F received fentanyl 2 μg/kg intravenously.
On the day of surgery, after ensuring adequate fasting status, children were premedicated with 0.5 mg/kg oral midazolam 30 minutes before induction. In the premedication room, baseline readings of Heat Rate (HR), SpO2, and Mean Arterial Pressure (MAP) were recorded. Intravenous access was obtained and children in Group F were administered fentanyl 2 μg/kg and children in Group D, dexmedetomidine 0.5 μg/kg intravenously. The calculated dose was diluted in 10 mL saline and given over 10 minutes as infusion. Then the children were transferred to the operation theatre and Electrocardiography (ECG), Non-Invasive Blood Pressure (NIBP) and pulse oximeter were attached. Glycopyrrolate 4 μg/kg intravenously was administered to children of both groups. All children were induced with thiopentone sodium 5 mg/kg intravenously. For tracheal intubation atracurium 0.5 mg/kg was administered and intubation done using endotracheal tube appropriate for the age and size. All children received paracetamol 10-15 mg/kg and ondansetron 0.1 mg/kg intravenously after intubation. Anaesthesia was maintained on O2 and N2O (66:33), isoflurane 0.2-1% and atracurium in all patients. At the end of surgery, anaesthetics were discontinued. Neuromuscular block was antagonised with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg intravenously once respiratory efforts ensued and extubation carried out when adequate tidal volume was achieved. Patients were kept in recovery position and monitored in the recovery room.
HR, MAP and SPO2 were noted in all patients immediately after discontinuation of anaesthetics and at 1, 3, 5 and 10 minutes after tracheal extubation. The time of discontinuation of anaesthetics was taken as time zero. The time intervals from discontinuation of anaesthetics to tracheal extubation and to eye opening in response to verbal stimuli were noted.
The quality of extubation was evaluated according to the 5 point extubation score [17]:
No cough, easy breathing
Minimal cough- 1 to 2 times, easy breathing (smooth extubation)
Moderate coughing (3 to 4 times)
Heavy coughing (5 to 10 times) and straining
Laryngospasm, severe cough >10 times
If there was no gross purposeful muscular movement, such as coughing, breath holding or laryngospasm within 1 minute just after tracheal tube removal, it was defined as smooth extubation [18]. Patients who developed a score of two and above were regarded as not having been extubated smoothly.
Sedation score was assessed at 5 and 10 minutes post-extubation using Ramsay sedation scale [19]:
Awake- Patient is anxious and agitated or restless or both
Patient cooperative, oriented, and tranquil
Patient responds to commands only
Asleep- Brisk response to light glabellar tap or loud auditory stimulus
Sluggish response to light glabellar tap or loud auditory stimulus
No response to light glabellar tap or loud auditory stimulus
Any complications like delayed recovery, undue sedation, cough, laryngospasm, bradycardia, hypotension, hypertension, nausea, vomiting were noted and treated appropriately.
Statistical Analysis
Statistical analysis of the data was done using SPSS software version 22. For quantitative parameters with normal distribution, Independent t-test was used to compare means between the study groups. Medians and Interquartile range (IQR) were compared between study groups using Mann-Whitney U test in case of non-normally distributed quantitative parameters. Chi-square test/Fisher’s-Exact test was applied to compare categorical outcomes between study groups (If the overall sample size was <20 or if the expected number in any one of the cells is <5, Fisher’s exact test was used.) A p-value of less than 0.05 was considered statistically significant.
Results
The patients in both groups were found to be comparable for demographic variables like age, weight and gender distribution. Duration of surgery as well as baseline haemodynamic parameters was also comparable between the two groups [Table/Fig-1].
Comparison of demographic variables and baseline haemodynamic parameters.
| Parameters | Group D Median (IQR) | Group F Median (IQR) | p-value |
|---|
| Age (years) | 7.5 (3) | 7 (3) | 0.756* |
| Weight (kg) | 24 (11) | 24 (12) | 0.413* |
| Gender M: F [N (%)] | 32:28 (53.3:46.6) | 27:33 (45:55) | 0.361† |
| Preoperative HR (beats/min) | 107 (16) | 109 (16) | 0.208* |
| Preoperative MAP (mm Hg) | 84.87 (12) | 83.10 (11) | 0.340* |
| Duration of surgery (min) | 48 (9.5) | 46 (14) | 0.477* |
*Mann-Whitney U test; †Chi-square test; p-value <0.05 considered significant
Extubation (5 point extubation score) was significantly smoother in group receiving dexmedetomidine with a median score of 2 (IQR 1) compared to group receiving fentanyl with a median score of 3 (IQR 1) (p-value <0.001) [Table/Fig-2].
| Extubation score categories | Group F (N=60) N (%) | Group D (N=60) N (%) |
|---|
| No coughing (1) | 0 (0%) | 24 (40%) |
| Minimal coughing (2) | 22 (36.67%) | 36 (60%) |
| Moderate coughing (3) | 38 (63.33%) | 0 (0%) |
The median time from discontinuation of anaesthetics to extubation showed a statistically significant difference between the two groups with median (IQR) time in group D being 10 (2) min and group F being 12 (4) minutes with a p-value of <0.001. The median time to eye opening was also significantly shorter in Group D, median IQR being 13 (4) minutes, while in Group F it was 14 (6.5) minutes with a p-value 0.040. Postoperative sedation score (Ramsay sedation score) was comparable between the two groups both at 5 and 10 minutes [Table/Fig-3].
Comparison of extubation parameters.
| Parameter | Group F | Group D | p-value |
|---|
| Time from discontinuation of anaesthetics to extubation (min): Median (IQR) | 12 (4) | 10 (2) | <0.001* |
| Time from discontinuation of anaesthetics to eye opening (min): Median (IQR) | 14 (6.5) | 13 (4) | 0.040* |
| Extubation score: Median (IQR) | 3 (1) | 2 (1) | <0.001* |
| Ramsay sedation score 5 min: (Mean±SD) | 2.85±0.659 | 2.93±0.666 | 0.490† |
| Ramsay sedation score 10 min: (Mean±SD) | 2.28±0.67 | 2.45±0.502 | 0.124† |
*Mann-Whitney U test; †Independent sample t-test; p-value <0.05 considered significant
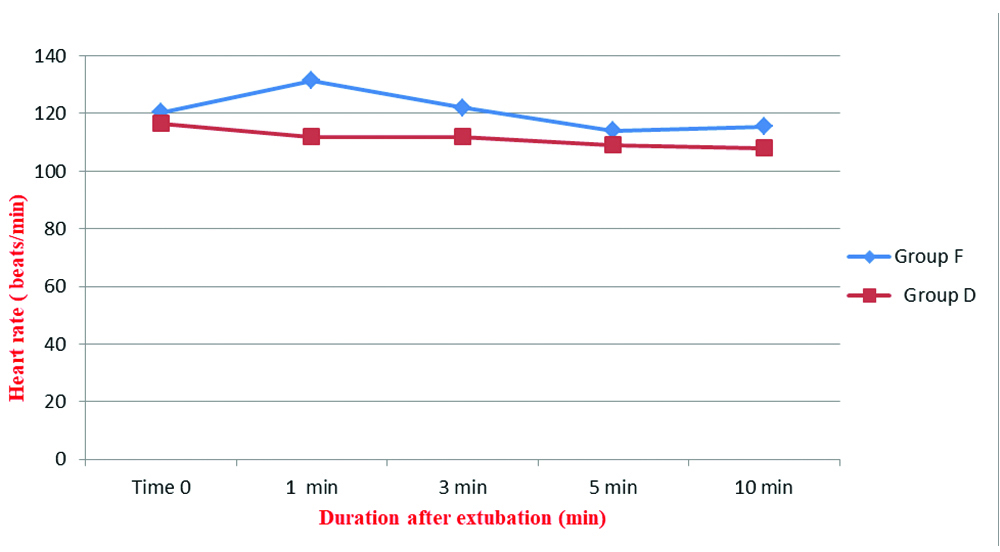
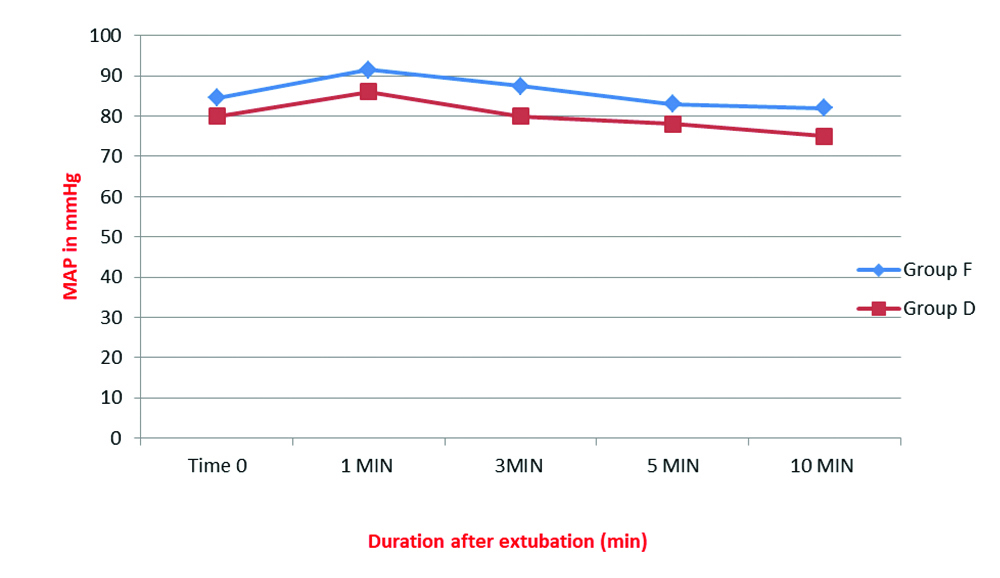
Extubation haemodynamics was better in the dexmedetomidine group. MAP and HR recorded immediately after discontinuation of anaesthetics and 1, 3, 5 and 10 minutes after extubation showed a significantly lower HR and MAP at all times in the dexmedetomidine group compared to fentanyl group [Table/Fig-4,5].
Comparison of Heart Rate (HR) after extubation; *p-value <0.001 at all monitored time intervals.
Mann-Whitney U test
Comparison of Mean Arterial Pressure (MAP) after extubation; *p-value <0.001 at all monitored time intervals.
Mann-Whitney U test
There were no episodes of any other side-effects like bradycardia, hypotension, nausea or vomiting in both the groups.
Discussion
Tonsillectomy and adenoidectomy has been associated with a high incidence of laryngospasm in the post-operative period [20]. Many children also present with Obstructive Sleep Apnoea (OSA) syndrome, where airway obstruction can pre-exist and an opioid free technique is found to be really appealing in the circumstances [21,22]. An awake but comfortable and settled child with minimum respiratory and airway compromise is desirable in the post-tonsillectomy period.
This study aimed to compare the effects of premedication using dexmedetomidine 0.5 μg/kg bolus with fentanyl 2 μg/kg on extubation characteristics. On analysing the results, it was observed that the children in the dexmedetomidine group had smoother extubation compared to fentanyl group. All the children were having minimal or moderate cough in the fentanyl group, whereas in the dexmedetomidine group 40% children had no cough at all. These findings were comparable with study by Turan G et al., who employed a similar extubation score to assess the quality of extubation in patients undergoing intracranial surgery where dexmedetomidine 0.5 μg/kg was used [17]. In a randomised controlled trial by Guler G et al., the effects of single dose dexmedetomidine on extubation after paediatric adenotonsillectomy were studied and it was agreed that agitation and pain scores in the dexmedetomidine group (0.5 μg/kg IV) was better than those in placebo group [23]. In the present study, the same dose was used, but as premedication and yielded similar results facilitating smoother extubation. Fan Q et al., also used a 5 point extubation quality score and found that dexmedetomidine 0.7 μg/kg produced smooth tracheal extubation after otologic surgery [24].
The mean time from discontinuation of anaesthetics to extubation and eye opening on verbal commands was significantly shorter in the dexmedetomidine group. These findings were concurrent with an earlier research conducted by Erdil F et al., comparing dexmedetomidine 0.5 μg/kg and fentanyl 2.5 μg/kg on emergence characteristics after adenoidectomy [16]. In a similar study by Sharma K et al., observed a mean extubation time of 7.70±1.62 minutes along with a decrease in mean duration of surgery in dexmedetomidine group compared to placebo, which was attributed to the decreased surgical bleed as a result of controlled hypotension and blunting of physiological responses by dexmedetomidine [25]. He XY et al., in a meta-analysis of randomised controlled trials comparing dexmedetomidine versus morphine or fentanyl, reported that dexmedetomidine was associated with a shorter time to eye opening and had similar efficacy to opioids in preventing emergence agitation in children [26].
In this study, dexmedetomidine provided more stable haemodynamics at extubation than fentanyl without any undesirable effects. HR, soon after discontinuation of anaesthetics as well as after extubation was significantly lower in the dexmedetomidine group than in the fentanyl group. MAPs also showed a significant reduction in the dexmedetomidine group compared to fentanyl. The reduction in haemodynamic response in the dexmedetomidine group were also noted by Di M et al., and Aksu R et al., and they concluded that the drug suppressed extubation stress response effectively [1,27]. Preinduction dexmedetomidine 1 μg/kg bolus in children undergoing adenotonsillectomy provided better haemodynamics and analgesia without any episodes of bradycardia or hypotension in the study by Sharma K et al., [25]. None of the children in both groups of the present study experienced any episodes of bradycardia or hypotension. It may be due to both the lower dose of drugs used along with the slow administration as an infusion over 10 minutes.
Postoperative sedation score (Ramsay sedation score) at 5 and 10 minutes after extubation were comparable between the two groups. The mean sedation score was higher in patients receiving dexmedetomidine, but there was no statistically significance. Sharma K et al., also had similar observations and opined that sedation produced by dexmedetomidine is advantageous in children [25]. In a study conducted by Prasad SR et al., among postoperative paediatric cardiac surgical patients, it was concluded that children given dexmedetomidine had earlier extubation and adequate sedation when compared with fentanyl [28]. In another study by Rani P et al., patients given dexmedetomidine were arousable but not awake, where as those who received fentanyl were mostly awake and the comparative increase in HR in the fentanyl group was attributed to this wake up state [8]. In the present study, children who received dexmedetomidine were lying comfortably and responding to commands.
There were no episodes of respiratory depression, nausea or vomiting noted in any children during the present study. Begum U et al., did not observe any complications in Post Anaesthesia Care Unit (PACU) such as apnoeic spells or desaturation episodes (SpO2 <90%). They also noted a significantly reduced incidence of postoperative nausea and vomiting with the use of dexmedetomidine in children receiving sevoflurane anaesthesia [11]. In a meta analysis Zhu M et al., concluded that dexmedetomidine provides safer sedation and analgesia compared to opioids, which are associated with respiratory depression [29]. They also found a decreased incidence of vomiting in children who received dexmedetomidine, which might be an advantage for airway safety. So dexmedetomidine can be an effective alternative to fentanyl in children, providing adequate level of sedation with no respiratory obstruction.
Limitation(s)
The study was not blinded and therefore there is possibility of an observational bias. Furthermore, five point extubation score and Ramsay sedation score were assessed by the researchers based on the subjective response of the study individuals, so there may be variability of responses elicited and it is difficult to standardise the variables.
Conclusion(s)
Premedication with single dose dexmedetomidine 0.5 μg/kg intravenously provided better quality of extubation and stable extubation haemodynamics, without undue sedation or any other side effects when compared to fentanyl 2 μg/kg. An opioid sparing approach including dexmedetomidine, as used in the study, may be followed in children undergoing tonsillectomy as well as other procedures where smooth extubation is preferred.
*Mann-Whitney U test; †Chi-square test; p-value <0.05 considered significant
*Mann-Whitney U test; †Independent sample t-test; p-value <0.05 considered significant