Global Malaria deaths reported by WHO in 2018 were concentrated in 20 countries in the Sub-Saharan Africa and India; of which 50% cases occurred in 5 countries namely Nigeria, Democratic Republic of the Congo, Mozambique, Uganda, and India, which accounted for 25%, 11%, 5%, 4% and 4%, respectively of world cases of malaria [1]. Delayed diagnosis is one of the major factors associated with death and hence early identification of the risk factors may help in limiting the morbidity and mortality due to malaria [2]. Malaria, which can be considered as tropical sepsis, may lead to multi organ failure [3,4]. Majority of patients of falciparum malaria may present with severe manifestations and complications including Multi Organ Dysfunction Syndrome (MODS) [5]. Vivax malaria can also lead to severe as well as resistant malaria like falciparum spp. and complications like thrombocytopenia, multi-organ failure and deaths are reported with P. vivax also [6-8].
In an ICU setting various scoring systems are used to determine prognosis of a patient. Some of the systems are Acute Physiology and Chronic Health Evaluation (APACHE) score, Sequential Organ Failure Assessment (SOFA) score, Simplified Acute Physiology Score (SAPS) and others [9-11]. APACHE score was introduced in 1981, which is generic and is not disease or organ specific. In APACHE, score is calculated in first 24 hours of ICU admission to predict mortality [9]. SOFA score, first introduced in 1994, is a dynamic and sequential ICU scoring system; mostly used for sepsis patients with organ dysfunction. It can also be used to prognosticate critically ill patients including patients of malaria [10].
The scoring system which has been solely used for malaria patients is MSS, developed by Mohapatra MK and Das SP in 2009 [12]. MSS is a disease specific prognostic scoring system which can predict the risk of hospital mortality due to falciparum Malaria in adults. Research on applicability of this prediction tool is lacking. MSS like APACHE II, SAPS II, and Mortality Prediction Model (MPM), require “one time” data. SOFA score and recently introduced q-SOFA score gives prediction based on the change occurring in organ function assessed serially or on day-to-day basis. As trend in recovery or dysfunction is depicted by this dynamic score it has better applicability in sepsis, tropical sepsis including malaria [13].
Hypothesis for this study was based on the assumption that MSS, devised for prediction of mortality of hospitalised patients of falciparum malaria; can give same prediction in patients that require ICU admission or are critically ill due to malaria (falciparum as well as with vivax). The present research was conducted to study the role of MSS in patients having malaria who were critically ill having multi organ dysfunction and to correlate the score with the risk of mortality.
Materials and Methods
This was a longitudinal study, conducted at a multispecialty teaching hospital located, in rural area of Vadodara, Gujarat. The study was conducted after obtaining approval from Institutional Ethics Committee of Sumandeep Vidyapeeth Deemed University (SVIEC/ON/MEDI/BNPG12/D123080). Patients who were admitted to ICU and emergency ward between January 2013 to May 2014, fulfilling inclusion and exclusion criteria were studied. The study was completed on 30 September 2014 (SVIEC/ON/MEDI/BNPG12/D14634).
Inclusion criteria: The present study included patients having smear positive malaria with multi organ involvement who were admitted in ICU and emergency ward. Patients of falciparum and vivax malaria were included.
Exclusion criteria: Patients in whom protocol of investigations/assessment that was required for MSS scoring like Glasgow Coma Scale(GCS), urine output, serum creatinine, blood urea, serum bilirubin, blood glucose, haemoglobin, platelet count, and total leucocyte count, could not be recorded properly, such patients were excluded. Patients below age of 18 years were also excluded.
Total of 60 consecutive patients who were fulfilling inclusion and exclusion criteria and were available between the study periods were taken. Out of these, 45 patients were admitted in ICU and 15 were admitted in the emergency ward. There were 27 patients of vivax, 30 of falciparum and 3 patients of mixed malaria infection.
In all patients, malaria was diagnosed by Field stain and with Giemsa stain. Both thick and thin smear were performed. Parasite count was done with plus system [14,15]. Laboratory investigations like Complete Blood Count (CBC), platelet count, malaria antigen test, whole blood clotting time, Prothombin Time (PT), Activated Partial Thromboplastin Time (APTT), arterial blood gas analysis, blood sugar, Renal Function Test (RFT), Liver Function Test (LFT), serum electrolytes and chest radiograph were performed on admission as per protocol. Daily assessment included GCS, urine output, respiration rate, heart rate, blood pressure and general as well as systemic examinations. Follow-up of survived patients was done till they were discharged from the hospital.
All selected patients were prognosticated with MSS; designed by Mohapatra MK and Das SP based on seven organ Systems (Neurologic, Renal, Haematologic, Hepatic, Respiratory, Cardiovascular, and Metabolic) and three levels of severity (I to III). Severity was judged as per validated abnormal physiological cut-off values/range prescribed in the scoring system [12]. Risk of mortality was calculated for each. The score was calculated on day of admission, day two and day seven. The score was analysed between two groups: “survivors” and “non-survivors”. “Probability of mortality” was defined as chance of probable death in relation to particular score obtained from MSS system. Mortality in present study patients was called “Actual Mortality”. ROC curve was prepared on data of worst MSS of three observations (of 0, 2 and 7th day). Specificity was plotted on X axis from 0 to 1 and sensitivity was similarly plotted on y axis by using pivot table in excel sheet.
Statistical Analysis
Data was entered in Excel, MS Office 13 from patient’s source file and compiled. Mean±SD and proportions were calculated, wherever applicable. Appropriate statistical tests were applied: t-test for two population proportions and Chi-square test for categorical values. The p-value <0.05 was considered as significant. ROC curve was plotted by calculating sensitivity and 1-specificity i.e., false positive fraction using data of worst MSS of first 7 days along with mortality by using pivot table in excel sheet. Area Under Receiver Operating Curve (AUROC) was calculated and values between 0.7- 0.8 was considered as an index for discrimination.
Results
Out of 60 patients, 43 were males and 17 females. Two groups were formed, 41 patients who survived formed the ‘survivors’ group and 19 patients that died formed the ‘non-survivors’ group. There were 30 males and 11 females in first group while the latter had 13 males and 6 females. Mean age of survivors was 38.56±2.27 and that of non-survivors was 40.21±5.6 years (p=0.718).
Out of 27 patients with P.vivax infection, 30 with P. falciparum infection and 3 with mixed infection, 18, 22 and 1 patients were in survivors group, respectively. On admission, 10 (16.67%) patients had 1+, 20 (33.33%) had 2+, 24 (40%) had 3+ and 6 (10%) had 4+ parasite count. There was no patient in 1+ parasite count group, two (10%) in 2+, eleven (45.8%) in 3+ and six (100%) in 4+ parasite count group. There were 28 patients in survivor group and 2 in non-survivor group who had 1 to 2 plus parasite count while 13 in survivors group and 17 in non-survivor group had 3 to 4 plus parasite count. There was statistically significant higher parasite count (of 3+ and 4+) in patients who died than that of survivors (p-value=0 .0031) [Table/Fig-1].
Shows mean age, gender and parasitic count in survivors and non-survivor groups.
| Malaria | Group 1(Survivors) N=41 | Group 2(Non-survivors) N=19 | Total*N=60 |
|---|
| Mean Age (years) | Male | Female | Total | Mean Age (years) | Male | Female | Total | Mean Age (years) | Male | Female | Total |
|---|
| P.Vivax | 37.47 | 14 | 4 | 18 | 39.03 | 6 | 3 | 09 | 39.03 | 20 | 7 | 27 |
| P. Falciparum | 39.98 | 16 | 6 | 22 | 39.61 | 5 | 3 | 08 | 39.72 | 21 | 9 | 30 |
| Mix | 48 | 0 | 1 | 01 | 38 | 2 | 0 | 02 | 39.27 | 2 | 1 | 03 |
| Total | - | 30 | 11 | 41 | - | 13 | 6 | 19 | - | 43 | 27 | 60 |
| Parasite count/Parasite Density in asexual parasite/microlit of blood | Group 1(Survivors) N=41 | Group 2(Non-Survivors) N=19 | TotalN=60 | |
| 1+ (50-500) | 10 | 0 | 10 (16.67%) | p-value=0.0031** |
| 2+ (500-5000) | 18 | 2 | 20 (33.33%) |
| 3+ (5000-50000) | 13 | 11 | 24 (40.00%) |
| 4+ (>50000) | 0 | 6 | 6 (10%) |
*Mean age of survivors was 38.56±2.27 and that of non-survivors was 40.21±5.6 years, which was not significant (p= 0.718).
**p-value was derived by comparing the combined parasite count of 1+ and 2+ (n=30) with parasite count of 3+ and 4 +(n=30)
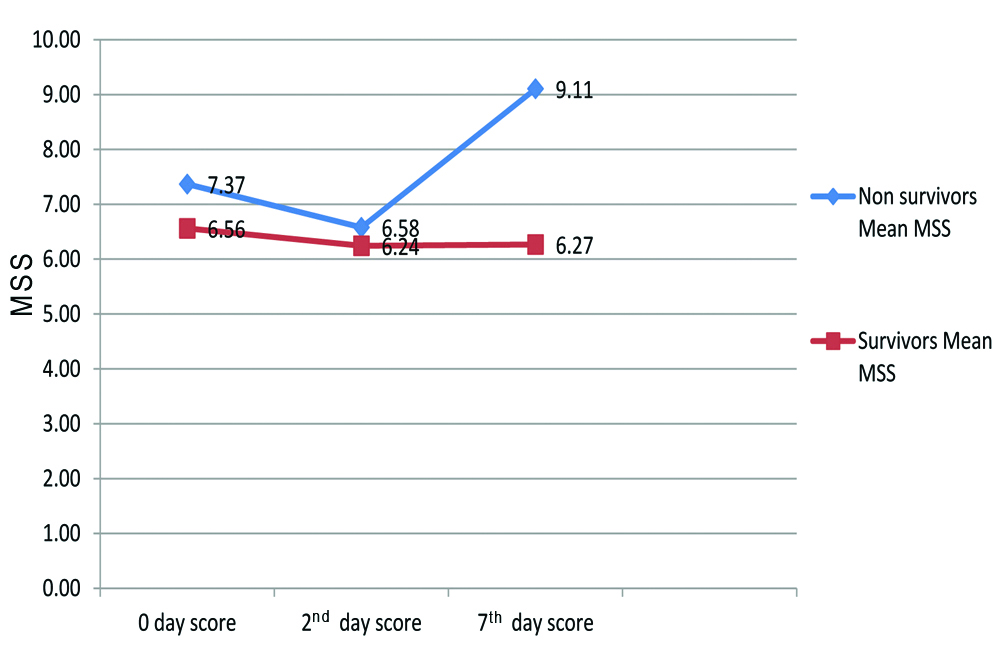
Mean MSS of all the three days is shown in [Table/Fig-2]. On day 0 and 2, Mean MSS was 7.37 and 6.58 in non-survivor group while it was 6.56 and 6.24, respectively in survivors group (p-value=0.291 on day 0 and p=0.581 on day 2). Mean MSS on day 7 was 9.11 in non-survivor group while 6.27 in survivor group, (p-value=0.005). As shown in [Table/Fig-2], there was a decreasing trend of mean MSS in both groups on day 2. On day 7, MSS increased in non-survivors while score remained almost same in survivors group.
Shows Mean MSS in survivors and non-survivor groups on day 0, 2 and 7.
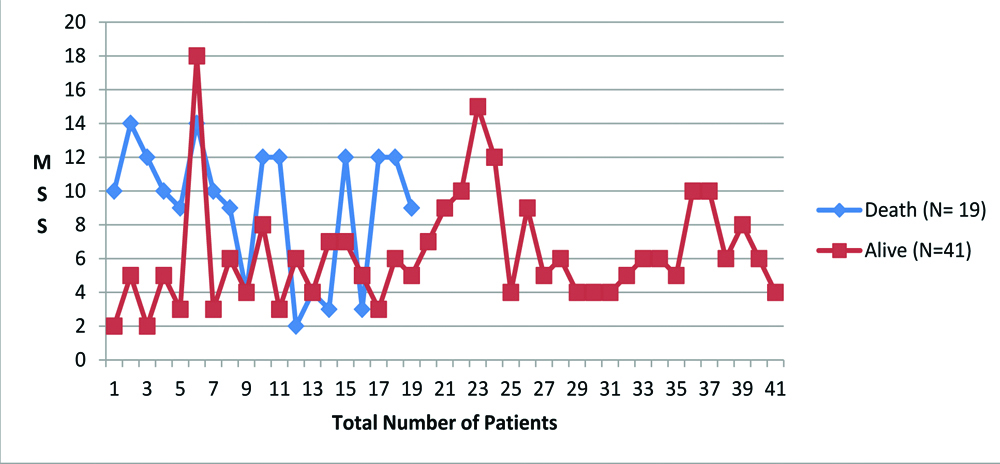
[Table/Fig-3] shows graph of MSS of day 7 of all sixty patients. As shown in this graph, MSS was higher in non-survivors than in survivors. Predicted and Actual mortality graph lines are seen to get approximated, which is not the case on day 0 (p=0.291) and 2 (p=0.581). Predicted mortality by MSS system when equated to actual mortality, was found to give better result based on MSS score of day 7 (p=0.005) [Table/Fig-4].
MSS score of all 60 patients on day 7.
MSS on day 0 (a), day 2 (b) and day 7 (c) related with predicted and actual mortality. On x-axis is MSS score and on Y axis is percentage of non-survivors.
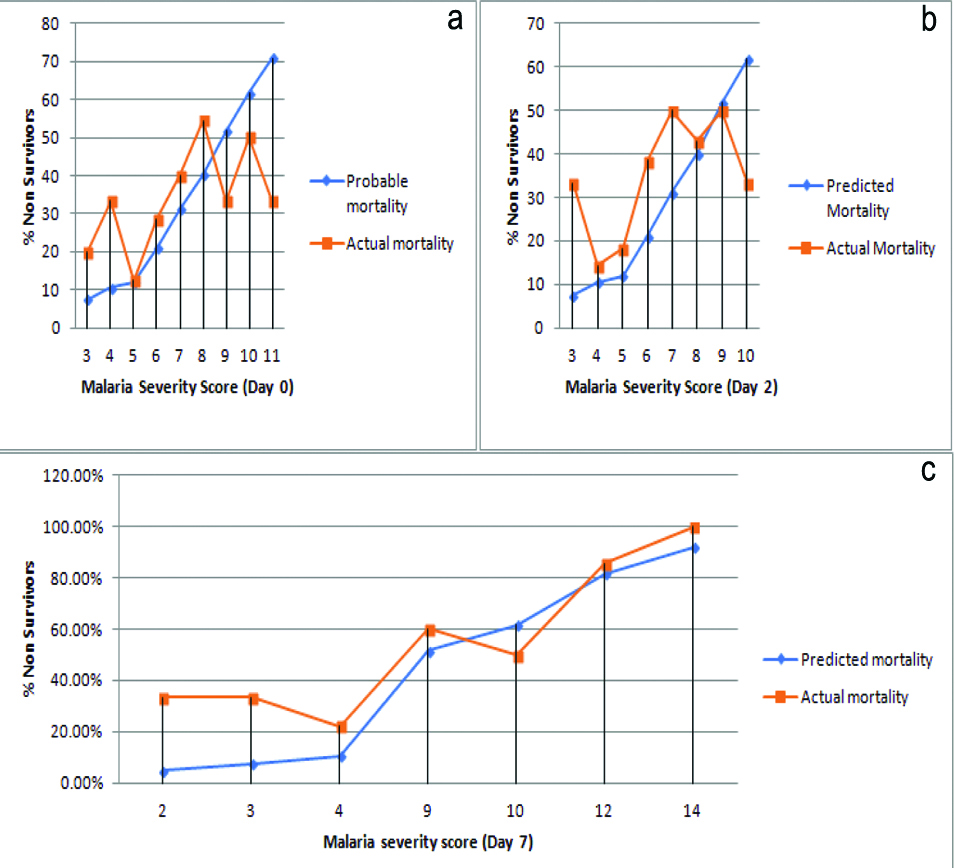
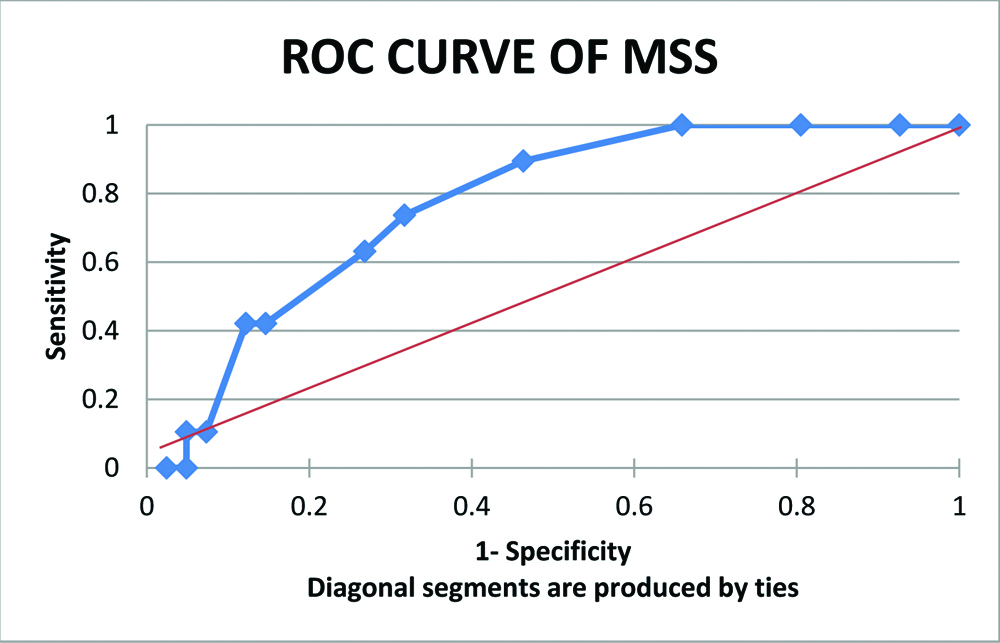
ROC curve of MSS shows that threshold on the curve that is close to the top left of the plot corresponds to MSS of 9. Hence, greater discriminant capacity was observed at MSS 9. Mean MSS in non-survivor group was 7.37 on day 0, 6.58 on day 2 and 9.11 on day 7. Thus MSS score of day 7 gave prediction reaching cut-off value of ≥9. AUROC was 0.76 with 95% confidence interval (0.65-0.88) [Table/Fig-5].
An ROC curve of Maximum MSS in 7 days of admission. AUROC- 0.769; (95% Cl, 0.65-0.88)
Discussion
In 2009, Mohapatra MK and Das SP devised a tool entitled “MSS” which is a validated and innovative method for assessing severity and assessing hospital mortality risk of falciparum malaria in adults. This prospective study was done in general wards of two medical College hospitals of Orissa, India [12]. Aim of the present research was applicability of MSS system in the setup, which is a rural-based medical college. Malaria is a common problem for which medical attention is needed. As MSS tool was devised by analysing patients from general hospital and that too of falciparum malaria, present study was designed to find out applicability in patients who were admitted in critical care wards with vivax and/or falciparum malaria. The authors also wanted to find out the applicability of MSS in severe vivax malaria admitted to ICUs.
Parasite count was a very important parameter in this study, patients having higher parasite density had increased risk of mortality. Admission parasite count of 3 to 4 plus was present in significantly higher number among non-survivors group than survivors. MSS was significantly higher in non-survivors on seventh day but not so on admission. For initial risk assessment of mortality and morbidity/MODS, parasitic count can be better prognostic marker while trend of MSS can predict mortality on further follow-up period. In a study conducted in Karachi, it was found that high parasite density was associated with severe clinical illness, complications and mortality [16]. Until now, studies had proved that P. falciparum had severe complications and mortality was greater. Complications like cerebral malaria, Disseminated Intravascular Coagulation (DIC), Acute Respiratory Distress Syndrome (ARDS), MODS etc., were reported in falciparum malaria [17]. Some recent studies found out that these complications were common in vivax malaria as well [18-20]. In this study, fatality rate was almost equal in both types; vivax and falciparum. Total 18 patients lived and 9 died amongst those suffering from P.vivax while 22 lived and 08 died in group of falciparum malaria. Three patients had mixed infection, of which 2 died.
In a study by Desai S and Lakhani JD, out of total 50 patients of sepsis in ICU, 12 patients of malaria had MODS in whom MSS and SOFA score were calculated. In this malaria group of 12 patients, 7 were alive while 5 died [4]. Mean MSS was 9.71 in survivors, while it was 12 in non-survivors. They concluded that MSS gives similar prediction like SOFA score. However, sample size of malaria patients were 12 only in this study [4].
In present study, mean MSS on day 0 and 2 was higher in non-survivors group however the difference was not statistically significant. On day 7, the statistical difference in MSS was noted between the groups. MSS in survivors and non-survivors show different trend as shown in [Table/Fig-2,4]. If treatment with anti-malarials and interventions for organ support was successful, decreasing/stationary trend was observed in scores. Thus, it can be implied that the scores increase when anti-malarials fail to act optimally (drug resistance or presence of dysregulated host response to malaria).
As per present study, serial scoring may show upward or downward trend, which is better prognosis indicator than an one-time severity assessment. In a similar type of study in patients of sepsis including that of malaria, serial measurement of SOFA score on third to seventh day (first week) was found to be a useful predictive tool than of day 0 (on admission) SOFA score and APACHE-II score [4]. APACHE score is not disease specific and takes into account other host factors like “Age” and “Co-morbidities” while MSS is a disease specific score but does not take into account of “Age” and “Associated co-morbidities” like APACHE, while prognosticating risk of death due to malaria. In present study, age was not significantly different in survivor and non-survivor group. Sequential measurement of MSS can be recommended as compared to one-time score measurement. Parasite count can be added in prognosis scoring system as it is the specific indicator for malaria severity.
On observing the trend of MSS in graph [Table/Fig-4] some patients of non-survivor group showed partial improvement in score on day 2, but again had increased score on day 7. This can be explained on the basis of partial beneficial effect of anti-malarials and organ-support management; however the scores may deteriorate subsequently due to anti-malarial drug resistance problem which is reported in vivax malaria also. Multi organ dysfunctions because of dysregulated host response in the form of new organ dysfunctions may come up subsequently. This may be due to inflammatory mediators and underlying circulatory, cellular and metabolic abnormalities [20].
This study was carried out in 45 patients of ICU and 15 patients of emergency ward. Any patients who were critically ill were admitted in ICU for the purpose of intensive management and organ support. Prognostic scoring system based on criteria laid down for organ dysfunction remains same or is influenced by type of hospital ward. This was the purpose of taking 15 patients from emergency ward also and not concentrating on ICU alone. There is a still a need for novel, definite and specific, severity and prognostic scoring system model derived through prospective work which can efficiently predict the outcome of any patient suffering from severe malaria admitted to critical wards.
Limitation(s)
Sample size of this study was small, however it was a longitudinal study unlike most prognostic scoring system based studies, which are retrospective ones. Malaria parasitic count was done with plus system, which may be less accurate. Patients recruited from ICU and also from emergency wards were not equal in number and were not compared. Some patients of malaria admitted in ICU had other co-morbidities also which had bearing with mortality.
Conclusion(s)
MSS was found to be useful prognostic score in severe falciparum/vivax malaria who needs intensive care treatment as sequential score giving significant difference in survivors and non-survivors on seventh day while parasitic count on day of admission can prognosticate mortality on admission also. Trend in MSS and inclusion of parasite count interwoven with seven organ dysfunction severity score can form a better malaria severity assessment tool.
*Mean age of survivors was 38.56±2.27 and that of non-survivors was 40.21±5.6 years, which was not significant (p= 0.718).
**p-value was derived by comparing the combined parasite count of 1+ and 2+ (n=30) with parasite count of 3+ and 4 +(n=30)