Rare Case of Intestinal-Type Adenocarcinoma Arising in Cervical Oesophageal Heterotopic Gastric Mucosa
Anuradha Sekaran1, Veena Pawar Vanere2, Sundeep Lakhtakia3, Mohan Ramchandani4, Duvuru Nageshwar Reddy5
1 Director and Chief Pathologist, Department of Pathology, AIG Hospitals, Hyderabad, Telangana, India.
2 Pathologist, Department of Pathology, AIG Hospitals, Hyderabad, Telangana, India.
3 Director of Endoscopy and EUS, Department of Medical Gastroenterology, AIG Hospitals, Hyderabad, Telangana, India.
4 Director of Interventional Endoscopy, Department of Medical Gastroenterology, AIG Hospitals, Hyderabad, Telangana, India.
5 Chairman and Chief of Gastroenterology, Department of Medical Gastroenterology, AIG Hospitals, Hyderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anuradha Sekaran, Mindspace Road, Gachibowli, Hyderabad-500032, Telangana, India.
E-mail: dr.sanuradha@aighospitals.com
Heterotopic Gastric Mucosa (HGM) also termed gastric inlet patch or inlet patch, is a rare and benign phenomenon in cervical oesophagus and can be missed during endoscopy. It has an average incidence of 2.5%. Adenocarcinoma arising in the background of gastric heterotopia is very rare and uncommon in the upper oesophagus. A 46-year-old male presented with hoarseness and progressive dysphagia for solids for the past one month. Upper Gastrointestinal Endoscopy (UGIE) revealed a tight stricture at 19-20 cm from the incisors. Initial mucosal biopsies were not conclusive. With high clinical suspicion of malignancy, patient underwent bougie dilation of oesophageal stricture followed by repeat biopsy. Histology revealed an intestinal type of adenocarcinoma, arising in a background of gastric heterotopia of the cervical oesophagus. Alcian Blue/Periodic Acid Schiff (AB/PAS) staining was positive in both the heterotopic glands and in the cancer, indicating the presence of intestinal metaplasia. Tumour cells were immunopositive for cytokeratin-7. A Positron Emission Tomography-Computed Tomography scan revealed a metabolically active lesion located in the upper third of the oesophagus along with uptake in right supraclavicular node. This case report describes a patient with primary intestinal type adenocarcinoma of the cervical oesophagus in the background of HGM not related to Barrett’s oesophagus.
Carcinoma, Dysphagia, Gastric inlet patch, Oesophageal stricture
Case Report
A 46-year-old male with no significant past medical or family history presented with chief complaints of sore throat and dysphagia to solid foods for duration of one year. He had no addictions, co-morbidities or loss of weight. His laboratory investigations were within the normal limits. On physical examination, with detailed examination of oral cavity, pharynx and neck no abnormalities were detected. Further workup included a barium swallow study which showed a persistent filling defect in the upper oesophagus extending from the inferior border of T2 to inferior border of T4. The remaining oesophagus appeared normal. No gastroesophageal reflux was detected when the patient was in the standing and lying positions. Bronchoscopy and endobronchial ultrasound showed no invasion of the airway structures. An UGIE with Endoscopic Ultrasound (UGIE-EUS) showed a tight stricture 19 to 20 cm from the incisors i.e., within 4 cm from the upper oesophageal sphincter [Table/Fig-1]. Endoscopic findings were highly suggestive of neoplastic lesion. Endoscopy guided biopsy was taken from the stricture area (19 to 20 cm from the incisors), however, the initial two biopsies showed only columnar-lined mucosa with focal gastric type of mucous glands. Possibility of gastric heterotopia was suggested on biopsy with no evidence of dysplasia/malignancy. As the clinical suspicion for malignancy was high, patient then underwent bougie dilation of oesophageal stricture followed by deeper biopsy. Histology revealed transition from benign squamous epithelium to HGM with dysplastic epithelial cells arranged in glandular pattern. Glands were closely placed, lined by cuboidal cells with vesicular to hyperchromatic nuclei and eosinophilic cytoplasm. Mild to moderate nuclear atypia with few mitosis. These dysplastic glands were infiltrating the lamina propria and within the splayed smooth muscle fibres.
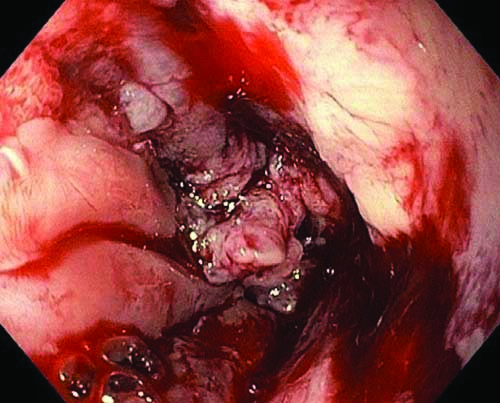
Upper gastrointestinal endoscopy with endoscopic ultrasound- A tight stricture 19 to 20 cm from the incisors.
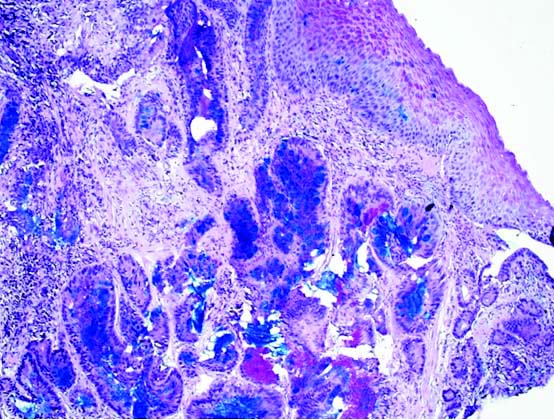
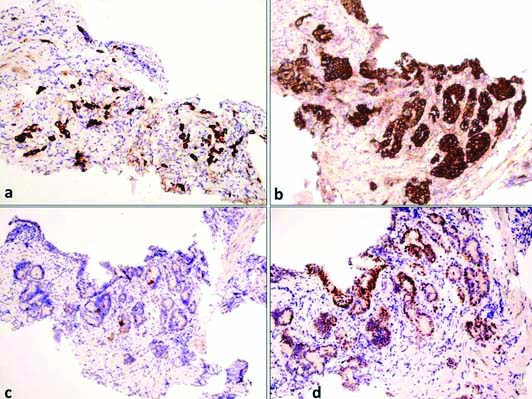
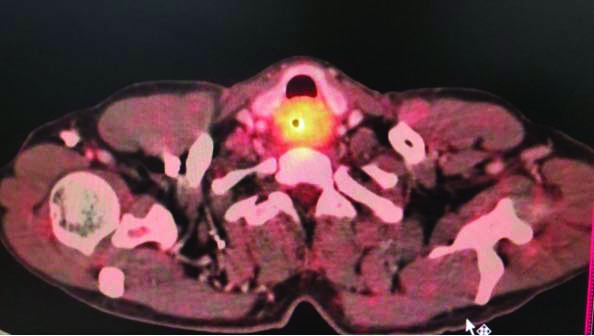
[Table/Fig-2] Differential diagnosis of adenocarcinoma in the background of Barrett’s oesophagus was ruled out based on the anatomical location of the lesion and presence of gastric heterotopia. In Barrett’s oesophagus, columnar epithelium extends from the stomach upto the gastroesophageal junction into the oesophagus. But in this case, the columnar epithelium was noted in the cervical oesophagus and was not in continuation with gastric mucosa. Staining for Helicobacter pylori (H.pylori) was also performed, but it was not detected in the cancerous lesion or in the surrounding normal epithelium either by H&E staining or by Giemsa staining. The sections were stained with AB/PAS. The cancer cells as well as the heterotopic glands were strongly positive for sulfomucin showing dark blue granules in the cytoplasm. This demonstrates the presence of acidic type of mucin (sulfomucin) due to intestinal type of metaplasia within the heterotopic areas [Table/Fig-3]. This shows that the subsequent adenocarcinoma arising from the heterotopic area is of intestinal phenotype. Immunohistochemistry was also performed, wherein the tumour cells were immunopositive for (cytokeratin) CK-7, MUC5AC and CDX2, and CK20 was negative [Table/Fig-4]. A Positron Emission Tomography-Computed Tomography (PET-CT) scan revealed a metabolically active lesion located in the upper third of the oesophagus extending at the level of C6 to T2, with appearance of metabolic activity with increase in size of right supraclavicular node [Table/Fig-5]. Rest of the body showed no uptakes. Fine Needle Aspiration (FNA) was not done as PET-CT scan findings were strongly indicative of metastasis.
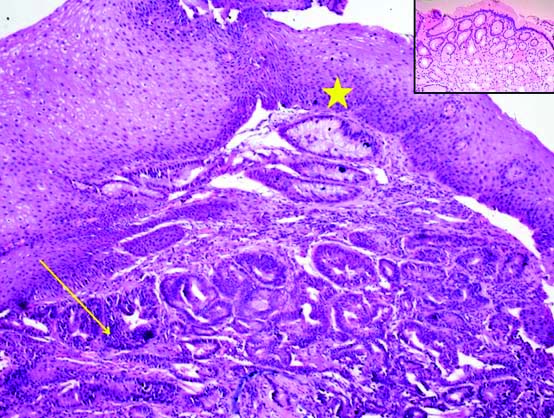
H&E stain, Adenocarcinoma with background Heterotopic Gastric Mucosa (HGM) and normal overlying squamous epithelium. Dysplastic tumour glands (arrow), HGM (arrow head) X200. Inset shows heterotopic gastric mucosa X100.
PAS/Alcian blue stain X200- dark blue colour highlights intestinal metaplasia.
Immunohistochemistry, a) CK7- Highlights tumour cells (X100); b) MUC5AC- Highlights tumour cells (X100); c) CK20- Negative in tumour cells (X100); d) CDX2- Nuclear positive in tumour cells (X100).
PET-CT- Metabolic active lesion involving upper third of oesophagus and in right supraclavicular lymph node.
Palliative chemotherapy was given and the oesophageal stricture was treated by bougie dilatation and oesophageal stenting. Patient has completed chemotherapy and is on follow-up from nine months and is doing well.
Discussion
The HGM of the proximal oesophagus is usually situated just beneath the upper oesophageal sphincter and recognised during UGIE [1]. The condition was first described by Schmidt in the cervical oesophagus [2]. The reported incidence of HGM in the cervical oesophagus varies substantially, ranging from 0.18 to 14%. Very few cases of intestinal type of adenocarcinoma arising within areas of HGM of the cervical oesophagus have been reported till date in comparison to adenocarcinoma of the distal oesophagus [3].
This wide range in incidence is due to the discrepancy between retrospective and prospective studies as many inlet patches are often neglected or unnoticed [4].
It usually occurs just 3-4 centimeters below the upper oesophageal sphincter, usually between 15 to 21 cm from dental arches seen on the laterals or the posterior surface of this area during careful withdrawal of the endoscope, and can easily be missed during a routine screening. It has been endoscopically described as a round to oval-shaped, flat, velvety pink or salmon in colour, sharply separated from the normal adjacent oesophageal mucosa, averaging 20 mm in size and can present itself with one or more lesions. Rarely, they can also be seen in the other, more distal parts of the oesophagus [5]. The clinicopathologic significance of inlet patches are receiving increasing recognition as the presence of acid (or mucus) secreting gastric tissue may predispose the individual to clinical symptoms which include bleeding, ulcerations, stricture, fistulation, formation of a ring or web or even malignancy, just like in our case.
There have been several theories in literature as to the development of cervical inlet patches out of which three mechanisms have gained prominence. The first is congenital hypothesis in that foetal oesophagus is lined by columnar epithelium. At 24 weeks of fetal gestation of embryo there is replacement of columnar epithelium in the middle part of oesophagus by squamous epithelium and it progresses towards the proximal and distal ends, as the proximal oesophagus is the last part to achieve the squamous epithelium, this process at time may not occur completely. This could explain the occurrence of heterotopic mucosa in proximal oesophagus [2]. Therefore, inlet patches may represent persistent embryological remnants [6].
The second theory is that it could be due an acquired condition secondary to irritation and injury of mucosa causing inhibition of proliferation of stem cells leading to metaplasia as occurs in patients with longstanding gastroesophageal reflux disease [7]. Another theory suggests that gastric heterotopia develops in a multi-stage process starting with occlusion of the proximal oeosphageal glands causing retention cysts with internal lining with columnar epithelium, luminal rupture of these cysts leads to areas of HGM [8].
A clinicopathologic classification has been proposed which categorises HGM into five distinct groups based on their clinical, endoscopic and histological characteristics [5]. Asymptomatic carriers of oesophageal HGM are classified as HGM I, symptomatic individuals complaining of odynophagia or dysphagia are classified as HGM II when endoscopic changes are absent. Patients with endoscopic features of oesophageal strictures, stenosis, webs or oesophagotracheal fistula are classified as HGM III. Patients with dysplasia (intraepithelial neoplasia) classified as HGM IV. Patients with invasive carcinoma are classified as HGM V. The patient in the case reported here had a category V complication (malignant transformation). The incidence of H.pylori occurring in a HGM is variable. Our case also did not show any positive H.pylori. In most cases, an HGM can be distinguished from Barrett’s oesophagus. Firstly, Barrett’s oesophagus is endoscopically well-characterised in that it is continuous with the distal oesophagus without circumferential interruption. And secondly, the histological presence of goblet cells (intestinal metaplasia) which is needed for the diagnosis of a Barrett’s, is not required for the diagnosis of an inlet patch. Although the location of the inlet patch was within the cervical oesophagus in this case, histology and special stains revealed the presence of intestinal metaplasia in this case and an inlet patch can occur more distally. “Double muscularis mucosae” is often seen in Barrett’s oesophagus. This was however not observed in this case. With regard to malignancies, it is usually squamous cell carcinoma which originates from the proximal third of the oesophagus and adenocarcinoma within the distal third of the oesophagus usually in association with reflux and Barrett’s metaplasia. Nonetheless, adenocarcinoma within the proximal oesophagus and unassociated to Barrett’s metaplasia are extremely rare and can arise either from a focus of HGM or from the oesophageal submucosal glands [9].
Several authors have suggested a link between adenocarcinoma arising from HGM with Barrett’s oesophagus. The metaplasia-dysplasia sequence occurring in Barret’s could also be the cause of adenocarcinomas arising in HGM, especially considering that HGM possibly contains parietal cells that secrete hydrochloric acid locally, and thus induce the metaplasia-dysplasia pathway. This case also showed intestinal metaplasia in a background of HGM, from which an intestinal-type adenocarcinoma had arose, which, being an even rarer phenomenon raised the possibility of a similar pathway of carcinogenesis in this tumour [10,11].
A panel of immunohistochemical stains such as MUC-1, MUC-2, MUC-5AC, MUC-6 and CDX2, in addition to the preliminary used stains CK7 and CK20 are recommended when adenocarcinomas arise in unusual locations, along with meticulous sampling of adjacent mucosa to confirm and differentiate between intestinal type and non-intestinal type adenocarcinomas since not only the detection but determining the exact type of adenocarcinoma may have implications for therapy.
The treatment options for symptomatic patients with an HGM include the use of a Proton-Pump Inhibitor (PPI) or histamine H2 receptor antagonists as an initial step. Endoscopic treatment using a variety of modalities is an option for symptoms unresponsive to PPI therapy or where there is evidence of preneoplasia or neoplasia. Benign symptoms are treated using argon plasma coagulation or radiofrequency ablation, whereas premalignant or malignant HGMs are treated with complete endomucosal resection of the patch or endoscopic submucosal dissection [12].
Conclusion(s)
Endoscopic diagnosis and reporting of inlet patches may be influenced by multiple factors, the most relevant being clinician’s awareness or due to their perception that an HGM may not be clinically relevant. Even when reported, HGM is considered a benign and rare phenomenon and does not warrant regular surveillance. However, patients with any mucosal abnormality visualised along with an HGM such as the presence of an upper oesophageal stricture or mass, should be kept under regular surveillance. Malignant progression in a HGM remains uncommon and specialist upper gastrointestinal surgeons/endoscopists should be aware of the neoplastic potential of these lesions. To conclude, this case highlights the potential clinical relevance of an HGM due to the rare incidence of a malignancy arising from it. Increased awareness and symptom correlation with endoscopy and histopathology findings for early therapeutic intervention is warranted.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 27, 2020
Manual Googling: Nov 20, 2020
iThenticate Software: Dec 21, 2020 (10%)
[1]. Harrison LJR, Kenwright D, Stringer MD, Esophageal heterotopic gastric mucosa in esophageal atresiaJournal of Pediatric Surgery Case Reports 2018 32:23-26.10.1016/j.epsc.2018.01.007 [Google Scholar] [CrossRef]
[2]. Camilo BA, Esperanza TT, Karen R, A case report of circumferential presentation with stricture of heterotopic gastric mucosa in the cervical esophagusRev Col Gastroenterol 2015 30(2):225-31.10.22516/25007440.45 [Google Scholar] [CrossRef]
[3]. Fashoyin A, Hartig G, Schelman WR, Ritter M, Agni R, Gopal DV, Aggressive adenocarcinoma of the cervical esophagus: Importance of a multidisciplinary approachCase Rep Gastrointest Med 2012 2012:82624610.1155/2012/82624623304576 [Google Scholar] [CrossRef] [PubMed]
[4]. Peitz U, Vieth M, Evert M, Arand J, Roessner A, Malfertheiner P, The prevalence of gastric heterotopia of the proximal esophagus is underestimated, but preneoplasia is rare-correlation with Barrett’s esophagusBMC Gastroenterol 2017 17(1):8710.1186/s12876-017-0644-328701149 [Google Scholar] [CrossRef] [PubMed]
[5]. Von Rahden BH, Stein HJ, Becker K, Liebermann-Meffert D, Siewert JR, Heterotopic gastric mucosa of the esophagus: Literature-review and proposal of a clinicopathologic classificationAm J Gastroenterol 2004 99(3):543-51.10.1111/j.1572-0241.2004.04082.x15056100 [Google Scholar] [CrossRef] [PubMed]
[6]. Tanaka M, Ushiku T, Ikemura M, Shibahara J, Seto Y, Fukayama M, Esophageal adenocarcinoma arising in cervical inlet patch with synchronous Barrett’s esophagus-related dysplasiaPathol Int 2014 64(8):397-401.10.1111/pin.1218125143128 [Google Scholar] [CrossRef] [PubMed]
[7]. Avidan B, Sonnenberg A, Chejfec G, Schnell TG, Sontag SJ, Is there a link between cervical inlet patch and Barrett’s esophagus?Gastrointest Endosc 2001 53(7):717-21.10.1067/mge.2001.11478211375577 [Google Scholar] [CrossRef] [PubMed]
[8]. Meining A, Bajbouj M, Erupted cysts in the cervical esophagus result in gastric inlet patchesGastrointestinal Endoscopy 2010 72(3):603-05.10.1016/j.gie.2010.05.00820801291 [Google Scholar] [CrossRef] [PubMed]
[9]. Riddiough GE, Hornby ST, Asadi K, Aly A, Gastric adenocarinoma of the upper oesophagus: A literature review and case reportInt J Surg Case Rep 2017 30:205-14.10.1016/j.ijscr.2016.11.01428086198 [Google Scholar] [CrossRef] [PubMed]
[10]. Latos W, Sieroń-Stołtny K, Kawczyk-Krupka A, Operchalski T, Cieślar G, Kwiatek S, Clinical evaluation of twenty cases of heterotopic gastric mucosa of upper esophagus during five-year observation, using gastroscopy in combination with histopathological and microbiological analysis of biopsiesContempOncol (Pozn) 2013 17(2):171-75.10.5114/wo.2013.3437623788986 [Google Scholar] [CrossRef] [PubMed]
[11]. Komori S, Osada S, Tanaka Y, Takahashi T, Nagao N, Yamaguchi K, A case of esophageal adenocarcinoma arising from the ectopic gastric mucosa in the thoracic esophagusRare Tumours 2010 2(1):e510.4081/rt.2010.e521139950 [Google Scholar] [CrossRef] [PubMed]
[12]. Maconi G, Pace F, Vago L, Carsana L, Bargiggia S, Bianchi Porro G, Prevalence and clinical features of heterotopic gastric mucosa in the upper oesophagus (inlet patch)Eur J Gastroenterol Hepatol 2000 12(7):745-49.10.1097/00042737-200012070-0000510929900 [Google Scholar] [CrossRef] [PubMed]