The Osteoarthritis (OA) is a very common disease among the elderly population and is associated with a lot of disability. A total of 60% patients with radiographic evidence of OA have symptoms and 15 to 30% patients present to practitioners with difficulty in ambulation due to OA [1]. Obesity is a major risk factor and prevalence of OA is also increasing day by day [2]. Primarily, OA histologically presents as synovial inflammation and clinical correlates of inflammation like joint swelling and effusion are seen [1]. OA is a degenerative disease of the joint characterised by hyaline articular cartilage loss focally and non-uniformly, sclerosis and increasing thickness of the subchondral plate due to joint margin showing osteophytic outgrowth, stretching of synovial capsule, mild synovitis and weakness of muscles bridging the joint [2]. As first line therapy for OA the NSAIDs are used mainly for symptomatic treatment but its use is limited by its adverse effects [3]. Therapy of OA today is palliative; no pharmacologic agent has been shown to prevent, delay the progression or reverse the pathologic changes of OA in humans. Although claims have been made that some NSAIDs have chondroprotective effect, adequately controlled clinical trials in humans with OA to support this view are lacking. NSAIDs do not have any effect on the disease process or does not inhibit or slow down the disease progression. Destruction of bones and cartilage is the hallmark of OA and rheumatoid arthritis. Disease modifying anti-rheumatic drugs or DMARDs are available for rheumatoid arthritis and considerable interest and inquisitiveness has developed for finding disease modifying drugs for OA in this study. There is a relationship between bone changes and articular cartilage damage in arthritis. Bone remodelling occurs by bone resorption and new bone formation. Many inflammatory mediators such as interleukins 1,6, tumour necrosis factor-α, nitric oxide, prostaglandins, influence bone formation and bone resorption [3]. Recent focus is to search for drugs that not only acts to relieve symptoms of OA but also aims at modifying the pathobiologic and pathoanatomic changes in articular cartilage. Many clinical studies on diacerein have shown its effectiveness to reduce the symptoms of OA [3]. In animal models of mouse granuloma model, it prevents cartilage breakdown [3,4] and in canine model of OA, slows the progression of cartilage leisons [3,5]. Many studies have shown that diacerein is completely metabolised to rhein in humans and in animals [3,6]. All the previous studies have shown that there are various methods by which diacerein and its active metabolite rhein acts but the exact mechanism for anti-inflammatory effects is not clearly known till date [3].
Earlier study have shown the effect of diacerein in acute inflammatory model in rats [3] and here chronic inflammatory model that is anti- arthritic effects of diacerein on FCA-induced arthritis in rats is studied. There is lack of sufficient study to show the effects of diacerein when used prophylactically to prevent the development of arthritis and so the present study was taken up to evaluate the anti-arthritic effects of diacerein when used prophylactically on FCA-induced arthritis in rats and to elucidate its possible mechanism of action.
Materials and Methods
This was an experimental study carried over a period of about two months in the Department of Pharmacology, SCBMC, Cuttack. The experimental protocol was approved by the Institutional Animal Ethical Committee (431/01/c/CPCSEA).
Albino rats of wistar strain have been used earlier in many studies to observe the anti-inflammatory and anti-arthritic properties of diacerein [3,7]. Hence, albino rats of wistar strain of either sex weighing between 100 to 200 grams [7] were procured and acclimatised under standard laboratory conditions in departmental animal house as approved by the committee (Registration Number is 431/01/c/CPCSEA for the purpose of Research and Experimention on animals, for one week prior to experiment). The animals were maintained on 22±3°C, under 12:12 hour dark and light cycles. Animals had free access to food and water. Experiments were carried out between 10:00 to 17:00 hours. After the experiments, the used animals were kept separately from others in animal house to enable observations for development of any complication.
Study Material:
The materials used in this experiment include weighing scale, plethysmometer, animal cages, mouth gag, syringe, high power microscope and X-ray apparatus.
The drugs used in the experiment were- FCA purchased from Sigma Aldrich (Each mL of FCA contains 1 mg of mycobacterium tuberculosis (H37Ra, ATCC 25177) heat killed and dried, 0.85 mL paraffin oil, 0.15 mL mannide mono oleate, Commercially available tablets of diacerein (Orcerin), Diclofenac sodium (voveran) (both the drugs were dissolved in normal saline and normal saline was used as control) and Ketamine.
To evaluate whether diacerein prevents arthritis and suppresses joint destruction in vivo, this prophylactic treatment model was followed where arthritis was induced on day 0 by FCA, and drug treatment was also started simultaneously from day 0 to day 21. Diclofenac sodium was used as standard anti-inflammatory drug for comparison [7], and normal saline was taken as control.
The animals were numbered, divided into 5 groups with 6 animals in each group and kept in separate cages and the cages were numbered. The basal body weight and hind paw volume of both right and left paw of all the animals noted on day 0 [8] and body weight and hind paw volume of both right and left paw of all animals noted on days 4th, 8th, 14th, 21st [3]. Grouping of animals was done. Different doses of diacerein (50 mg/kg,100 mg/kg, 200 mg/kg) [3], diclofenac (5 mg/kg) or vehicle (normal saline 1 mg/mL) were administered orally once daily from day 0 to day 21.
Normal control group (Group I): Animals in this group were treated with normal saline solution per oral [9].
Diclofenac treated (Group II): at dose of 5 mg /kg [4]
Diacerein treated (Group III): at the dose of 50 mg/ kg [4]
Diacerein treated (Group IV): at the dose of 100 mg/kg [3]
Diacerein treated (Group V): at the doses of 200 mg/kg [3]
Study was undertaken to investigate the antiarthritic effect of diacerein in prophylactic treatment model of adjuvant induced arthritis. On day 0 animals were anaesthesied with ketamine (120 mg/kg) [10], adjuvant induced arthritis was produced by sub plantar injection of 0.05 mL of FCA, into the plantar surface of right hind paw [8].
Assessment Parameters:
1. Arthritic assessment:
To analyse the course of the disease and study the effect of the drugs on adjuvant induced arthritis in rats, the swelling of the adjuvant injected hind paw was determined on day 0 and the development of arthritis as indicated by increasing paw volume measured by volume displacement by plethysmograph and also the body weight were measured on 4th, 8th, 14th, and 21st day to confirm the reduction in arthritis
a. Paw Volume evaluation in mL:
Paw volume was calculated on days 0,4,8,14,21 days by means of plethysmometer. Mean change in injected and noninjected paw oedema was recorded with respect to the basal paw volume, and percentage inhibition of paw oedema with respect to the control group was calculated as: The measured paw volumes are expressed as:
Mean change in paw volume from basal on the respective days and percentage inhibition of paw volume from control.
% Inhibition of oedema with respect to untreated group is given by the formula:

Where i=% inhibition of paw oedema
ΔV Treated=mean change in paw volume of treated rat
ΔV untreated=mean change in paw volume of untreated rat [11].
b. Body weight:
Basal body weight of each animal was measured with the help of precalibrated weighing balance, before injecting CFA. Mean body weight in grams and percentage change in body weight from basal calculated for the respective days (4th, 8th, 14th, 21st).
2. Radiographic assessment of joint damage
Radiographic assessment and evaluation of joint damage.
After completion of the study period on day 22, the animals were anaesthetised with ketamine and placed on the X-ray plate. X-ray of both right and left ankle joint of each rat from control, diacerein and diclofenac treated groups done and compared. X-ray apparatus (NE-X ray machine 100MA, Japan operated at 220 V with a 54 V peak, 0.2 s exposure time was used to take the radiographs.
3. Histopathological analysis
On day 22, rats were anaesthetised and sacrificed by using ether [3]. Paws of rats were dissected out for histopathological examination. Sections were fixed in 1% formalin, decalcified, sectioned and stained with hematoxylin and eosin to study the histopathological changes in all the groups under light and high-power microscope.
Statistical Analysis
The data were analysed using Statistical Package for the Social Sciences (SPSS) version 20. Paired and unpaired t-test was done. A p-value of less than 0.05 was considered to be statistically significant.
Results
Maximum inhibition of paw volume with diclofenac 5 mg/kg in the Freund’s complete adjuvant induced arthritic paw volume was from 8th to 14th day and with diacerein maximum inhibition of Freund’s adjuvant induced arthritic paw volume was on the 21st day with 100 mg/kg [Table/Fig-1].
Change in paw volume in adjuvant induced arthritis in rats in prophylactic model by drugs.
| Groups | Drugs dose (mg/kg) | Mean change of right hind paw volume from basal (in mL)±SEM(%Inhibition of paw volume from control) |
|---|
| Baseline | 4th day | 8th day | 14th day | 21st day |
|---|
| I (Control) | Normal Saline(1 mL/kg) | 1.11±0.03 | 1.86±0.02 | 1.11±0.03 | 0.55±0.04 | 0.2±0.06 |
| II (Standard) | Diclofenac 5 mg/kg | 1±0.01 | 0.65±0.10*** (65.16) | 0.04±0.03*** (96.4) | 0.016±0.01*** (97.09) | 0.03±0.04** (83.5) |
| III | Diacerein50 mg/kg | 1.06±0.02 | 0.31±0.03*** (83.04) | 0.13±0.03 (88.09) | -0.01±0.01*** (102) | -0.06±0.03** (130) |
| IV | Diacerein100 mg/kg | 1.05±0.02 | 0.21±0.04*** (88.5) | 0.01±0.04*** (100) | -0.29±0.09* (154.36) | -0.56±0.15** (380) |
| V | Diacerein200 mg/kg | 1.03±0.02 | 0.2±0.03 (84.9) | 0.05±0.03*** (95.52) | -0.05±0.03*** (109) | -0.4±0.012** (300) |
n=6, Statistical analysis done from Control Values by Unpaired t-test, *p<0.05, **p<0.01, ***p<0.001
There was a decrease in body weight due to Freund’s adjuvant in normal saline treated group from 4th to 21st day of observation. In the diclofenac treated group there was also a greater decline in body weight from 4th to 21st day, in contrast in the diacerein treated group there was an increase in body weight from 4th to 21st day in all the three doses [Table/Fig-2].
Effect of drugs on body weight in adjuvant induced arthritis in rats in prophylactic model.
| Groups | Drugs dose (mg/kg) | Mean body weight (in gm)±SEM(% change in body weight from baseline) |
|---|
| Baseline | 4th day | 8th day | 14th day | 21st day |
|---|
| I (Control) | Normal Saline (1 mL/kg) | 129.5±2.47 | 128±2.4 (-0.54) | 127.5±2.2** (-1.54) | 125.3±2.2*** (-3.24) | 123.8±2.2*** (-4.4) |
| II (Standard) | Diclofenac 5 mg/kg | 114±6.8 | 112.1±6.9*** (-1.75) | 109.08±6.5*** (-4.31) | 100±6.1*** (-11.99) | 98.66±6.5** (-13.45) |
| III | Diacerein 50 mg/kg | 90.33±4.9 | 91.16±4.9* (0.09) | 85.38±11.2** (5.4) | 95.5±5.17*** (5.72) | 96.83±5.05*** (7.19) |
| IV | Diacerein 100 mg/kg | 78.9±4.97 | 79.08±4.71 (0.22) | 77±2.5 (2.4) | 80.1±3.07*** (1.52) | 81.71±3.3 (3.56) |
| V | Diacerein 200 mg/kg | 94.1±3.8 | 94.83±3.9* (0.77) | 96.16±3.80 (2.18) | 97.33±3.71*** (3.43) | 98.16±3.55*** (4.31) |
n=6, Statistical analysis done from Basal Values by paired t-test, *p<0.05, **p<0.01, ***p<0.001
On day 22 normal rat paw showed no soft tissue swelling with normal joint space and no increased opacity or sclerosis [Table/Fig-3], but adjuvant induced arthritic paw showed soft tissue swelling, sclerosis of bone around joint, increased opacity and the joint space is barely visible with reduction of joint space [Table/Fig-4].
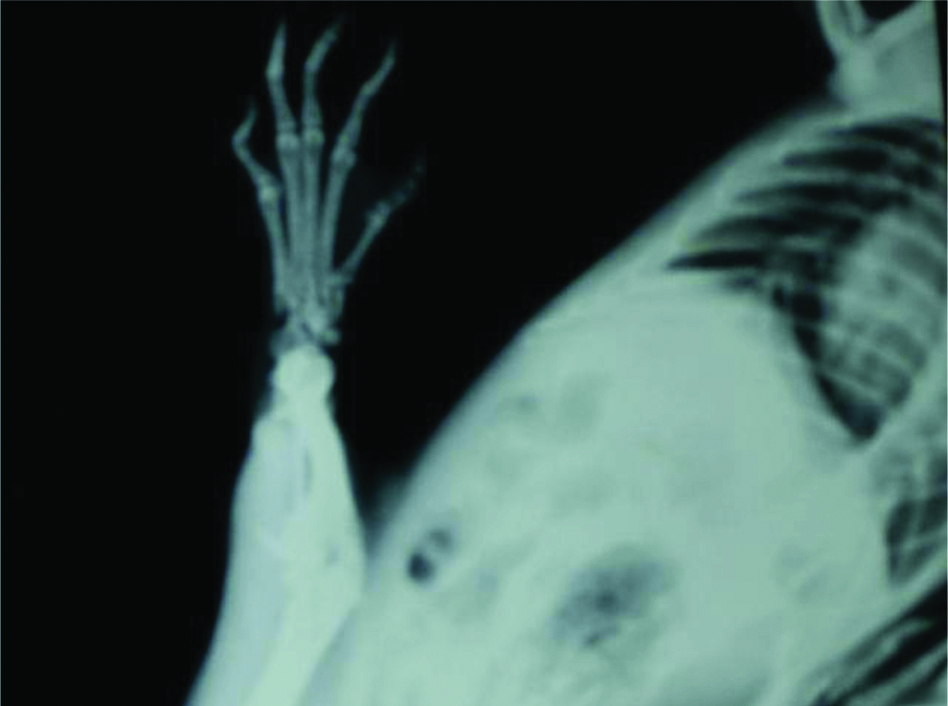
Radiograph of normal rat paw on day 22.
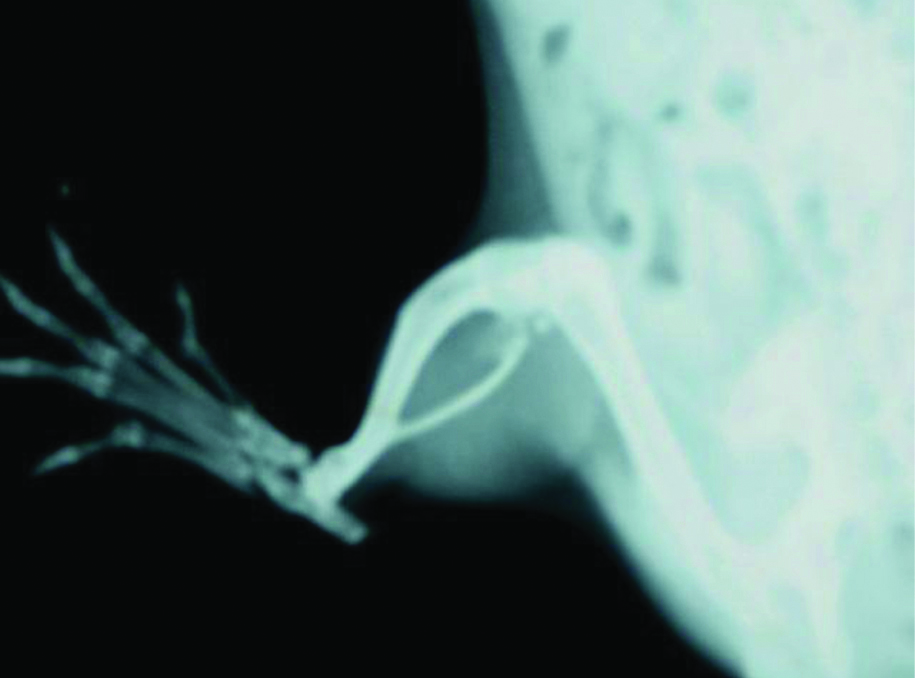
Radiograph of adjuvant induced arthritic paw on 22nd day.

Similarly, on day 22 Diclofenac 5 mg/kg treated rat paw showed soft tissue swelling, grossly dcreased joint space, increased opacity of joints showing bony sclerosis [Table/Fig-5] but on day 22, treatment with Diacerein 100 mg/kg it was observed that soft tissue swelling was markedly less, joint space is normal and no increased opacity or bony sclerosis or features of new bone formation seen [Table/Fig-6].
Radiograph of Diclofenac 5 mg/kg treated rat paw on 22nd day.
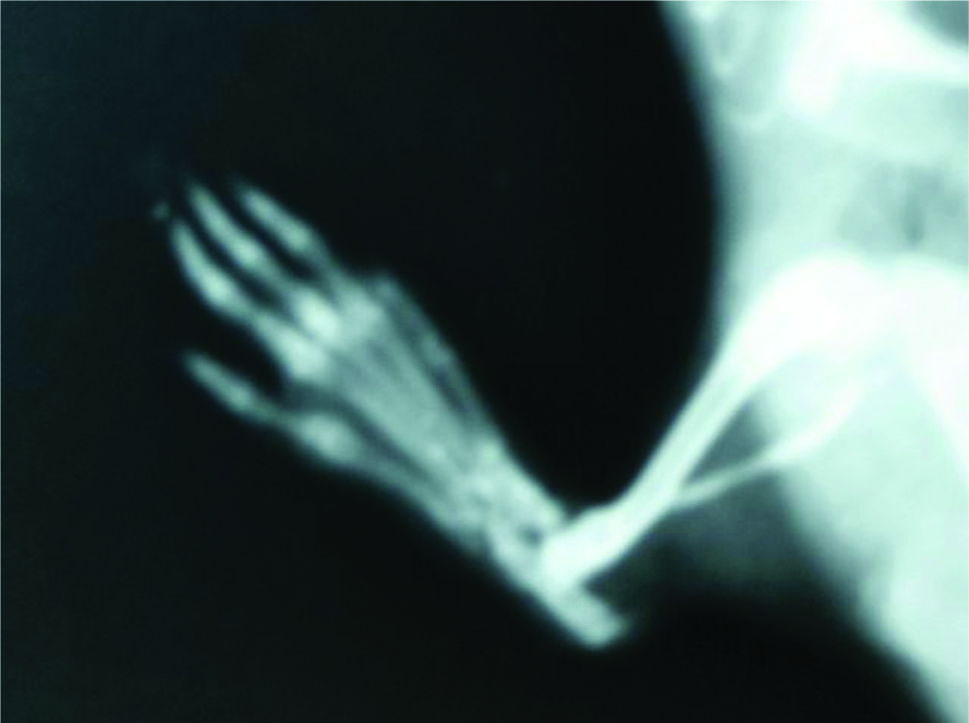
Radiograph of Diacerein 100 mg/kg treated rat paw on 22nd day.
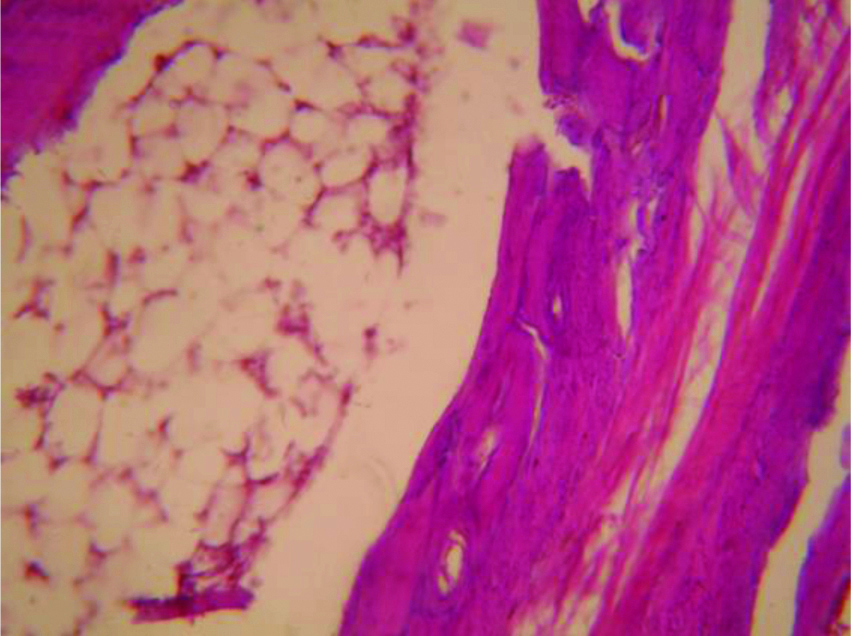
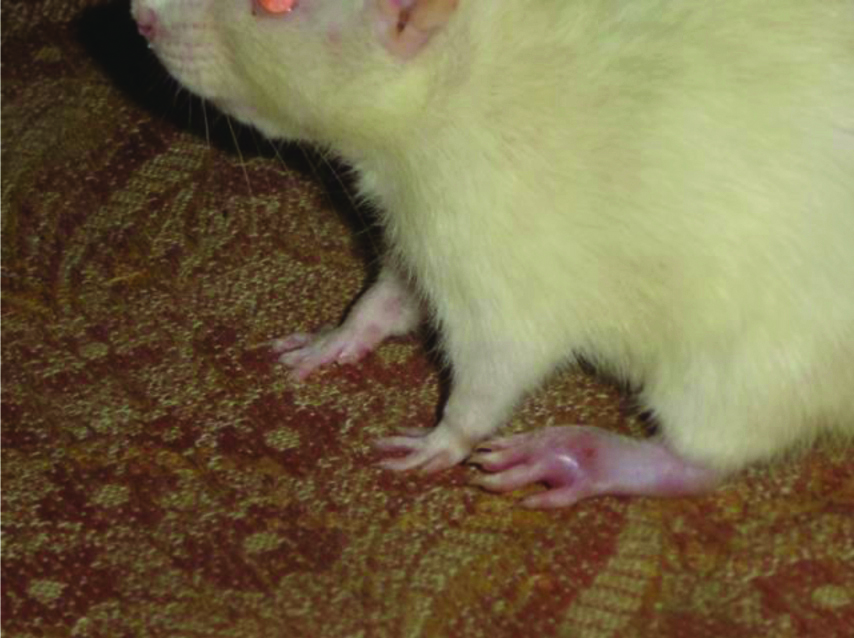
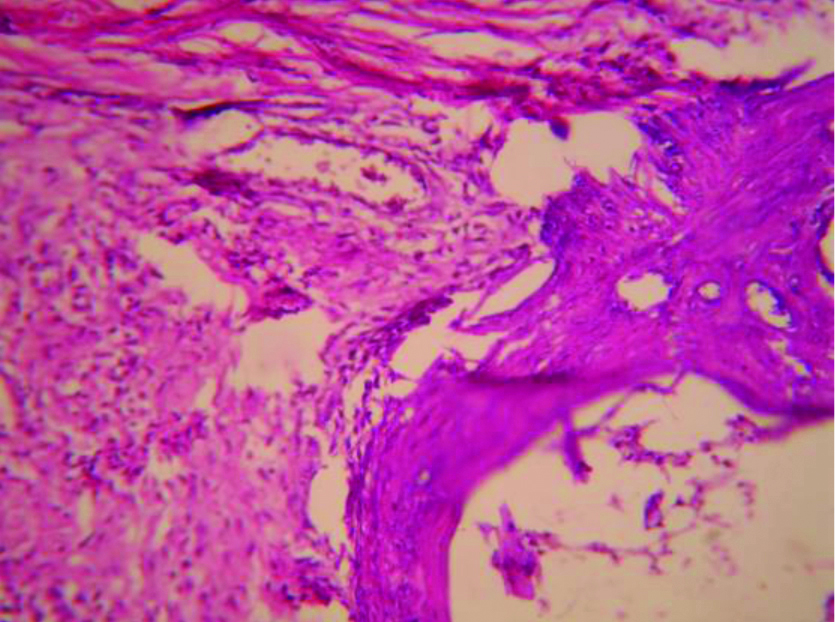
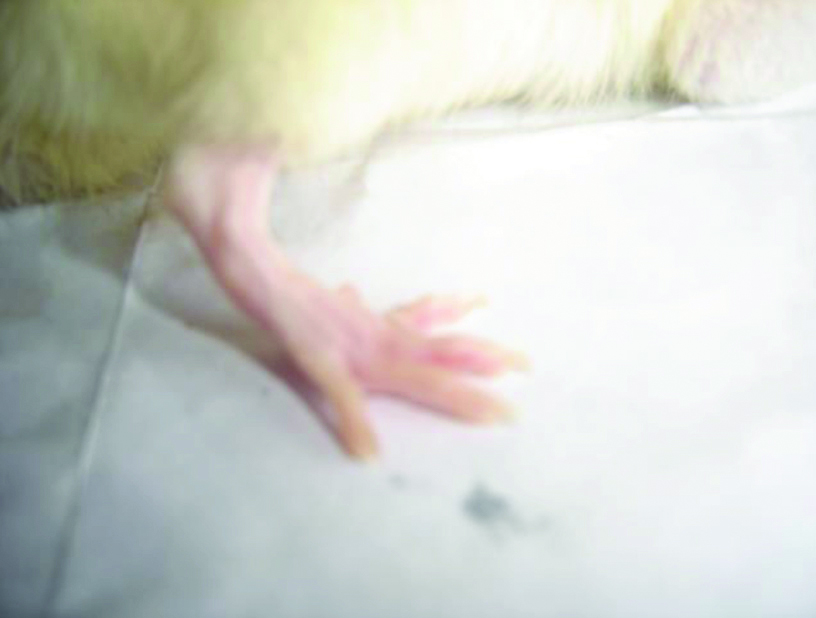
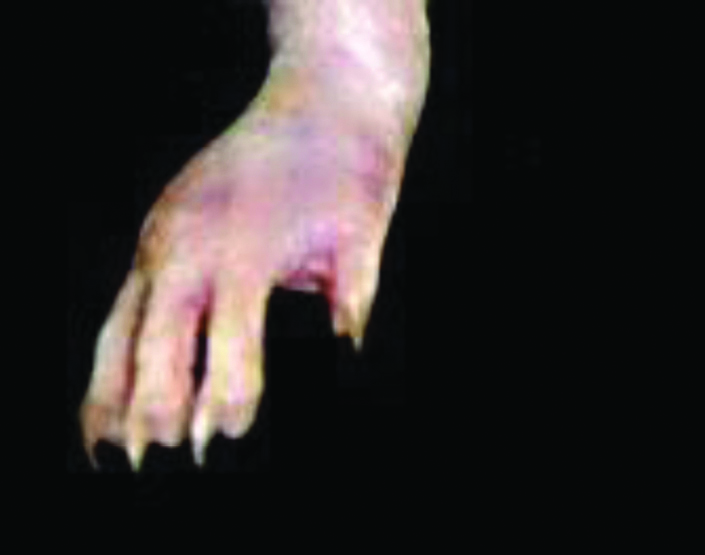
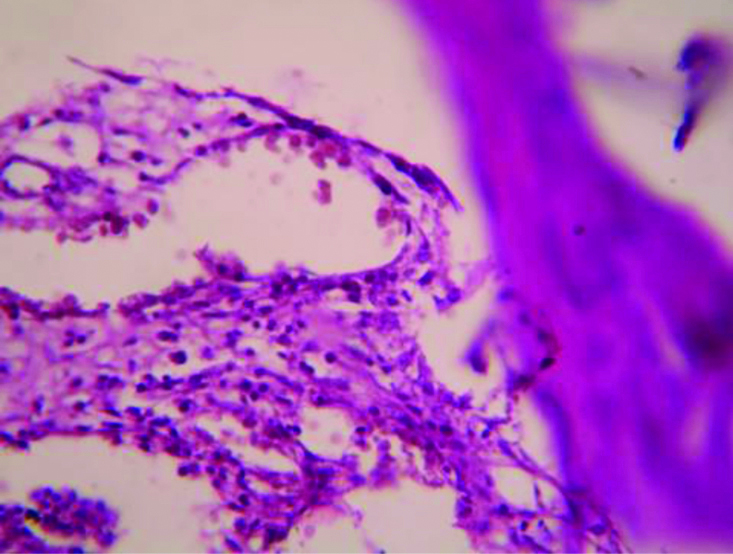
The picture of normal rat paw [Table/Fig-7]. On histopathologic examination with Haematoxylin and eosin staining and 40x magnification under microscope, normal bone marrow with adipocytes observed. Bony spicules visible with no inflammatory infiltrate and bone layer is intact [Table/Fig-8]. Picture of adjuvant induced arthritic rat paw on day 22 [Table/Fig-9] and histopathology of the same joint shows disruption of bony spicule margin, surrounding the bony spicule there is a zone of inflammatory cells and the bony layer is thickened at places and may denote bony sclerosis [Table/Fig-10]. The picture of diacerein 100 mg/kg treated rat paw on 22nd day [Table/Fig-11] and on taking histopathology section of the same it was seen the cartilaginous lining is intact, intact bony spicule was visible with no surrounding zone of inflammation, very little inflammatory infiltrate and Bone marrow is normal [Table/Fig-12]. Picture of diclofenac 5 mg/kg treated rat paw [Table/Fig-13], the histopathologic section of which shows surface of bony layer is somewhat disrupted and surrounding the bony spicule is a layer of inflammatory infiltrate with a lot of inflammatory cells [Table/Fig-14].
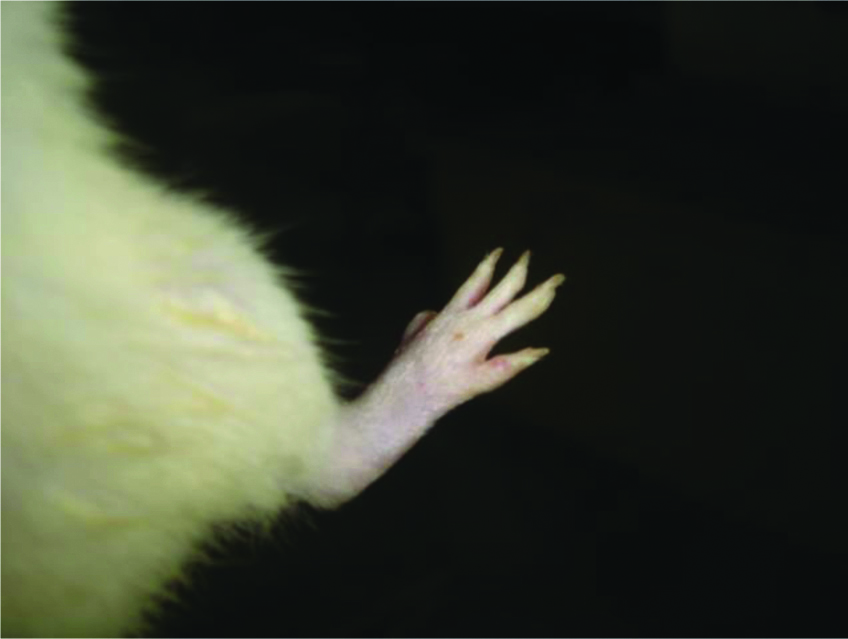
Histopathological section of normal rat paw (X400).
Adjuvant induced arthritic rat paw on 22nd day.
Histopathologic section of adjuvant induced arthritic rat paw on 22nd day (X100).
Diacerein 100 mg/kg treated adjuvant induced arthritic rat paw on 22nd day.
Histopathologic picture of Diacerein 100 mg/kg treated adjuvant induced arthritic rat paw on 22nd day (X100).
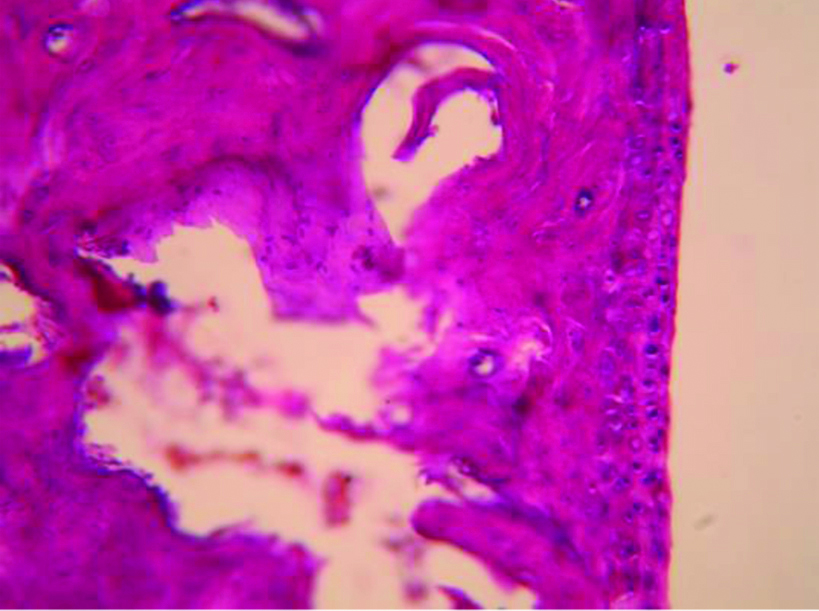
Diclofenac 5 mg/kg treated rat paw on 22nd day.
Histopathologic picture of Diclofenac 5 mg/kg treated rat paw on day 22nd day (X400).
Discussion
Osteoarthritis is not merely a disease of cartilage but all the tissues of the joint, including the synovium, subchondral bone, periarticular muscle and supporting ligaments are affected in the disease [12,13]. Cartilage damage is a crucial feature of OA and is generally considered irreversible. Accordingly drugs that block the destruction of cartilage will be of therapeutic value. Currently the primary approach in the clinical treatment of OA involves the use of NSAIDs, and hyaluronan, which provide symptomatic relief but provide no apparent disease modifying effect [14,15]. The increasing incidence of arthritis and the inadequacy of present day drug therapy underline the need to improve the technical feasibility of discovering safe and effective anti-arthritic agents [16]. Diacerein is an effective treatment of OA [17]. The action of diacerein is linked to mechanisms that are not yet been completely clarified. The present study on animal model was taken up to further obtain insight into the anti-arthritic properties of diacerein. In earlier studies diacerein in doses of 50,100, 200 mg/kg have shown analgesic, anti-inflammatory and anti-arthritic properties in rats. Diacerein, specifically its active diacetyl derivative rhein, is an IL-1 inhibitor. Rhein is an anthraquinone found in plants of the genus Cassia and has moderate anti-inflammatory and analgesic activity and weak laxative effects [18,19].
The form of arthritis produced by injection of microbial particles into rats is called adjuvant induced arthritis. It was first described by Pearson in 1956. It was induced by killed mycobacterium and mineral oil. After about 10 to 14 days inflammation appears in the ankles, wrists, tarsals, interphalangeal joints of rats. This disease is not limited to the joints but has extra-articular manifestations, including tendonitis, iritis, nodular lesions in the visceral organs, urethritis and diarrhoea. The symptoms peak after 10 to 15 days after injection followed by slow resolution. The synovial lesions when viewed under the microscope are similar to those observed in human rheumatoid arthritis. The rat adjuvant induced arthritic model is widely used for the evaluation of NSAID drugs and anti-rheumatic drugs [3]. Adjuvant induced arthritis is the only experimental model which has been considered to be as parallel to human arthritic disease more closely than any other laboratory model [8,20]. Diacerein when administered from day 0-21 in doses of 50 mg/kg, 100mg/kg significantly reduced the arthritic paw volume starting from 4th day to 21st day of observation while diacerein 200 mg/kg produced this effect from 8th to 21st day of observation [Table/Fig-1]. Diclofenac 5 mg/kg significantly reduced the paw volume from 4th day of observation to 21st day of observation [Table/Fig-2]. Maximum percentage inhibition of paw volume was seen with diacerein 100 mg/kg at 21st day of observation while with diclofenac it was seen on 14th day of observation [Table/Fig-1]. Similar findings have been reported by Tamura T et al., who found out that when diacerein was administered orally for three weeks to rats with adjuvant induced arthritis, it significantly inhibited the swelling of the injected and non injected paws at doses of 100 mg/kg [3]. In this study, all the three doses of diacerein increased the body weight of rats significantly in comparison to basal from 14th to 21st day which diclofenac failed to do. In the control as well as the diclofenac 5 mg/kg treated group a significant decrease in body weight was observed from 14 to 21 days [Table/Fig-2]. It has been mentioned in earlier studies that change in body weight have been used to assess the course of the disease and the response to therapy of anti-inflammatory drugs [21]. As the severity of arthritis increased, the changes in the body weight of rats also occurred during the course of the experimental period. This fact of the loss of body weight during the arthritic condition is also supported by earlier observation on alteration of the metabolic activities of the diseased rats. The increased body weight during treatment of diacerein may be due to restoration of absorption capacity of intestine [22].
Limitation(s)
Less number of animals was used in the study, the effect of diacerein in combination with NSAIDs treatment has not been evaluated mainly because NSAIDs are most often not used prophylactically for arthritis but could have been done, the effect of diacerein on the biochemical parameters of arthritis has not been evaluated.
Conclusion(s)
FCA is an effective antiarthritic model which produces progressive increase in paw volume and reduction in body weight as a part of systemic involvement. So this antiarthritic model was rightly used. As seen in this study diacerein in all the three doses when used orally for three weeks as prophylaxis showed significant antiarthritic property as evident by inhibiting the swelling of FCA injected paw as well as causing increase in body weight in comparison to basal values. Diacerein possesses antiarthritic and anabolic properties as observed in the animal models and so can be a promising disease modifying drug for OA. Further studies can be conducted to ascertain its role as a disease modifying drug for OA.
n=6, Statistical analysis done from Control Values by Unpaired t-test, *p<0.05, **p<0.01, ***p<0.001
n=6, Statistical analysis done from Basal Values by paired t-test, *p<0.05, **p<0.01, ***p<0.001