Preterm neonates are defined as those born before 37 weeks. The anatomical features of preterm neonates vary from that of an adult, such that they have a large tongue, an anterior funnel shaped larynx, angled vocal cords and an omega shaped epiglottis. Their physiological development also differ as they have a greater risk for apnoea of prematurity, airflow obstruction, bronchopulmonary dysplasia, low functional residual capacity and decreased transient oxygen tension. Congenital airway anomalies have a prevalence rate of 0.2-1 in 10,000 live births. Congenital Tracheal Stenosis (CTS) caused by complete tracheal rings is one of the rarest forms of all the airway anomalies that occur, which is characterised by presence of complete ‘O’ shaped cartilaginous rings of trachea devoid of a membranous part. The case discussed in the article is that of a day 26 preterm neonate with increasing abdominal distension and failure to thrive. The neonate was posted for an urgent exploratory laparotomy for abdominal decompression and ileostomy. Patient was kept on Continuous Positive Airway Pressure (CPAP) support for four days immediately after birth; however, intubation was never attempted nor required. With a history of respiratory depression and lack of history of previous intubation, difficult airway cart was kept ready prior to surgery. Supraglottic Airway (SGA) devices were at the core of airway rescue in this case of undiagnosed CTS. This case report shows how and why a preparation for difficult airway in preterm neonates is a necessity. It will provide as a guide if a similar case is encountered by the anaesthetist.
Case Report
A 26-day-old male, 1.5 kg preterm neonate (born at 34 weeks by normal vaginal delivery with delayed cry) presented with complaints of abdominal distension, poor feed intake and bilious aspirate.
The patient developed respiratory distress soon after birth and was admitted to the Neonatal Intensive Care Unit (NICU) on day 2 of life. In the NICU, the baby was put on CPAP support for 4 days; after which the patient required oxygen supplementation via Hudson mask and nasal prongs for 7 days. He was stable on CPAP for the period of respiratory depression and had no history of any intubation attempt or requirement. Following this, he had stable vitals on room air. The patient showed no respiratory complications after day 11 of life. The other systems of the body were normal. The patient was then shifted to a medical ward from NICU from where the parents took discharge against medical advice.
The baby then developed jaundice with a total serum bilirubin value of 16.9 mg/dL on day 15 of life and was admitted in the present institute for medical care. Phototherapy was started for 3 days, following which, he was started on dopamine infusion at the rate of 2 ug/kg/min for hypotension with Noninvasive Blood Pressure (NIBP) reading of 40/20 mmHg. The NIBP on Dopamine infusion was 49/27 mmHg. Sepsis work up was done in order to rule out any component of infection associated multi-system derangement. It revealed an increased value of C-Reactive Protein (CRP) (18.5 mg/L) and hence, the baby was started on broad spectrum antibiotics. At the same time, a COVID-19 testing was done, the result of which was negative.
Baby started showing signs of improvement as CRP reduced to 6.4 mg/L and the total serum bilirubin also dropped down to a value of 11 mg/dL. The baby was put on formula feeds on day 20 of life. He was then kept in NICU on antibiotics, intravenous fluids and feeds for the next 6 days. However, the baby then developed abdominal distention on day 26 of life which was associated with bilious aspirate and jaundice. An X-ray abdomen was done. The findings were suggestive of necrotising enterocolitis.
The baby was initially managed medically with the help of broad spectrum antibiotics and IV fluids and did not require any supplementary oxygen at the time. Following this, he was posted for exploratory laparotomy in view of distended abdomen, failure to take feeds with suspected subacute obstruction and failure to thrive. There were no signs of respiratory depression and he was maintaining saturation of 100% in room air. Difficult airway cart was kept ready with I gel, Laryngeal Mask Airway (LMA), oral airway, bougie and stylet. Dopamine infusion was continued at the minimal rate of 2 μg/kg/min owing to sepsis induced hypotension.
The patient was suspected to have subacute obstruction of the bowels; exploratory laparotomy was mainly planned for diagnostic purposes and to decompress the abdominal distension and create a stoma in ileum. Monitors were attached and fluid infusion (2% dextrose with Ringer’s lactate at the rate of 20 mL/kg/hr) started through IV. Patient was preoxygenated for 3 minutes on closed paediatric circuit with oxygen flow of 5 L/min. Dopamine infusion was continued throughout the perioperative period.
The neonate was premedicated with Inj. Atropine 20 μg/kg and Inj. Fentanyl 2 μg/kg. The patient was induced with Inj. Propofol 3 mg/kg and after confirmation of ventilation, Inj. Atracurium 1 mg/kg was administered. Intubation was undertaken with a No. 0 Miller Laryngoscopic blade under direct laryngoscopic view and Endotracheal tube no. 3 (uncuffed). Under direct laryngoscopic view, vocal cords were visible; however, endotracheal tube could not be passed distally beyond the cords. Similar single attempts were made each with a number 2 and 2.5 uncuffed endotracheal tube; but the endotracheal tubes could not be maneuvered beyond the vocal cords even with screwing action. Additional airway manipulation of airway was avoided in view of occurrence of airway trauma and a medical nature of disease with urgent and not an emergency nature of the surgery. As the patient was desaturating with SpO2 values between 75-80%, intubation attempts were aborted and patient was ventilated with a mask on closed circuit. An ENT surgeon was immediately called to perform direct laryngoscopy. Meanwhile, patient was ventilated sufficiently with bag and mask ventilation on closed as well as Jackson Rees (JR) circuit. 0° and 30° laryngoscopy was performed to reveal stenotic trachea along its entire length. The patient was given Dexamethasone 0.1 mg/kg and Hydrocortisone 2 mg/kg in order to reduce the oedema caused by multiple intubation attempts and to avoid bronchospasm. An I-gel no.1 was then inserted for effective ventilation till the effects of muscle relaxant wore off. As the effects of anaesthetic drugs wore-off, the patient started breathing spontaneously with good respiratory attempts and no signs of respiratory depression.
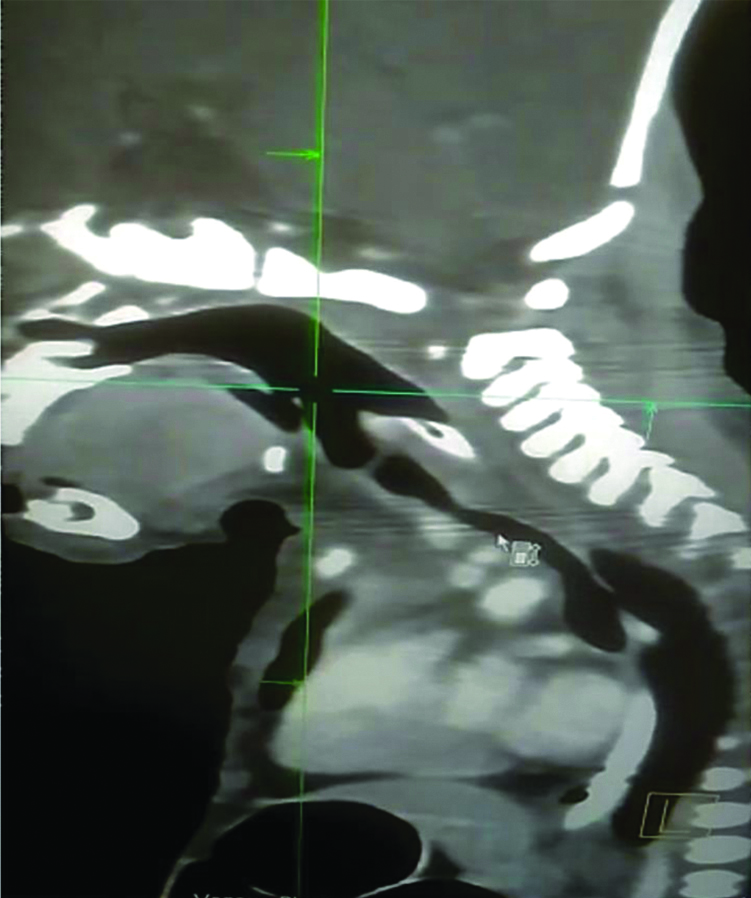
The patient was shifted to NICU after he was completely awake and crying. The surgery was postponed and further CT neck and chest was advised for the patient which revealed and confirmed the diagnosis of CTS caused by complete tracheal rings [Table/Fig-1]. As a result of the findings and absence of frank obstruction, the surgery was deferred. The baby was posted for a planned tracheoplasty in the coming days. However, the patient succumbed to septic shock two days later.
CT scan showing lateral view of narrowed trachea (shown by pointer) suggestive of Congenital Tracheal Stenosis (CTS).
Discussion
The anatomical features of preterm neonates vary from that of an adult, such that they have a large tongue, an anterior funnel shaped larynx, angled vocal cords and an omega shaped epiglottis. Their physiological development also differ as they have a greater risk for apnoea of prematurity, airflow obstruction, bronchopulmonary dysplasia and decreased transient O2 tension [1,2]. They have a high oxygen consumption rate (6 mL/kg/min), low functional residual capacity and a high carbon dioxide production rate (100-150 mL/kg/min) [3].
Congenital airway anomalies have a prevalence rate of 0.2-1 in 10000 live births; with each airway anomaly requiring a unique approach for management of the same [4]. Preterm neonates are at a greater risk of suffering from congenital malformations. These individuals have a transitional circulation and a unique airway anatomy. They are vulnerable to adverse respiratory and cardiac events with high occurrences of apnea and resultant desaturation. Anaesthetic agents have an unfavourable neurological, developmental and cardio-respiratory impact. The presence of a comorbidity increases the risk of surgery in these patients [5].
There are multiple types of congenital airway anomalies seen in neonates which include choanal atresia, laryngomalacia, sub-glottic stenosis, vocal cord paralysis, tracheomalacia, bronchogenic cyst, tracheal stenosis: of which laryngomalacia is the most common [6]. The airway anomalies can be differentiated into obstructing airway lesions; further divided into intrinsic (cysts, webs), extrinsic (mediastinal tumours, vascular rings or slings) or abnormal development (CTS); and communicating airway lesions (laryngotracheal clefts and tracheoesophageal fistulae) having an atypical connection between oesophagus and the airway [7]. These anomalies arise from maldevelopment in the embryonic, pseudoglandular, canalicular, saccular and alveolar phases of respiratory system development. Neonates with airway anomalies can present with clinical symptoms like irregular breathing, wheezing, sleep apnoea and difficulties with breast feeding. Computed Tomography (CT), Magnetic Resonance Imaging (MRI) combined with bronchoscopy and bronchography are the investigations carried out for the anomalies [8].
CTS is a rare life-threatening condition. It consists of a gamut of stenotic airway lesions that mainly comprise of complete tracheal rings. Complete Tracheal Rings are generally classified into three types- segmental, funnel-like, generalised hypoplasia [9]. The clinical presentation of CTS is caused by complete tracheal rings ranges from being asymptomatic to being severely symptomatic. In asymptomatic patients, the condition is mostly diagnosed incidentally, later in childhood with occurrence of respiratory infections. Neonates, if symptomatic, may present with cough, wheeze, stridor, retractions, cyanosis, and sometimes require ventilatory support. The patient may also suffer from failure to thrive. The symptoms may be exacerbated by inflammation and airway manipulation. A direct or fibre optic laryngoscopy with CT scan is diagnostic of CTS [9].
CTS is a rare condition which can result in life threatening respiratory complications in patients. It is mostly diagnosed during infancy and are commonly associated with symptoms of respiratory obstruction. CTS rarely shows an isolated presentation and a 50% tracheal narrowing is easily tolerated by paediatric population [10]. Tracheoplasty is the surgical treatment of choice for CTS. This can be combined with tracheal stents for better outcomes. The prognosis postsurgery has improved tremendously with a long-time survival rate of 88% in patients [7].
The patient discussed above had history of respiratory depression after birth for which the neonate required noninvasive ventilation in the immediate postnatal period. Following this, the patient had minimal oxygen requirement and was kept on nasal prongs at 2 L/min for seven days. There was no requirement for intubation with invasive ventilation anytime during the period of life of the baby. The patient showed no signs or symptoms of respiratory obstruction after day 11 of life and was active and crying on room air. Although the preoperative anaesthesia check-up did not reveal any signs suggestive of airway anomaly, the difficult airway cart was kept ready for any incidence of unpredictable difficult intubation.
In a similar case described by Dwivedi D et al., difficult airway cart was kept ready with straight and curved blade laryngoscopes, SGA devices (LMA and I-gel) [11]. As the patient was getting ventilated, intubation was attempted but was not successful. Suspecting an airway abnormality in the index patient, the ENT surgeons examined the patient and diagnosed the patient with stenosis of the entire trachea caused by ‘O’ shaped tracheal rings. Thus, a SGA device (I-gel) was used. As per the Unanticipated Paediatric Difficult Intubation Guidelines (2016), suggested by the All India Difficult Airway Association (AIDAA) [12], supraglottic device was used as a rescue measure for unimpeded ventilation as described in the second step of secondary intubation plan.
As multiple intubation attempts were made, steroids (dexamethasone and hydrocortisone) were given to avoid airway oedema and bronchospasm. Tracheostomy could not be attempted as entire length of trachea appeared to be stenosed. A decision was taken by the surgeons to postpone the surgery as it was not a frank obstruction and obtaining a safe secure airway gained precedence. In another case scenario described by Kundal R et al., a similar airway anomaly was encountered [13]. However, the surgery required in that neonate was an emergency and thus the patient was intubated with a feeding tube and attached to JR circuit.
Conclusion(s)
Unanticipated difficult airway in paediatric patients can have adverse consequences and sometimes even cause mortality in patients if not managed in time. Being a rare undiagnosed congenital anomaly, there is not much awareness about CTS and complete tracheal rings. Challenges like difficult airway management, bronchospasm associated with the complications linked with administration of anaesthesia in neonates are seen in this patient. The following case report and certain other similar difficult airway scenarios encountered suggest that the availability of a supraglottic device as suggested by the AIDAA is a must in any paediatric surgical case.
[1]. Karnati S, Kollikonda S, Abu-Shaweesh J, Late preterm infants. Changing trends and continuing challengesInternational Journal of Pediatrics and Adolescent Medicine 2020 7(1):38-46.10.1016/j.ijpam.2020.02.00632373701 [Google Scholar] [CrossRef] [PubMed]
[2]. Stein ML, Park RS, Kovatsis PG, Emerging trends, techniques, and equipment for airway management in pediatric patientsPaediatr Anaesth 2020 30(3):269-79.10.1111/pan.1381432022437 [Google Scholar] [CrossRef] [PubMed]
[3]. Harless J, Ramaiah R, Bhananker SM, Pediatric airway managementInt J Crit Illn Inj Sci 2014 4(1):65-70.10.4103/2229-5151.12801524741500 [Google Scholar] [CrossRef] [PubMed]
[4]. Varela P, Torre M, Schweitzer C, Nakamura H, Congenital tracheal malformationsPaediatr Surg Int 2018 34(7):701-13.10.1007/s00383-018-4291-829846792 [Google Scholar] [CrossRef] [PubMed]
[5]. Lee JH, Zhang J, Wei L, Yu SP, Neurodevelopmental implications of the general anesthesia in neonate and infantsExp Neurol 2015 272:50-60.10.1016/j.expneurol.2015.03.02825862287 [Google Scholar] [CrossRef] [PubMed]
[6]. Daniel SJ, The upper airway: Congenital malformationsPaediatr Respir Rev 2006 7(Suppl 1):S260-63.10.1016/j.prrv.2006.04.22716798587 [Google Scholar] [CrossRef] [PubMed]
[7]. Mok Q, Airway problems in neonates- A review of the current investigation and management strategiesFront Pediatr 2017 5:6010.3389/fped.2017.0006028424763 [Google Scholar] [CrossRef] [PubMed]
[8]. Berrocal T, Madrid C, Novo S, Gutiérrez J, Arjonilla A, Gómez-León N, Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathologyRadiographics 2004 24(1):e1710.1148/rg.e1714610245 [Google Scholar] [CrossRef] [PubMed]
[9]. Hofferberth SC, Watters K, Rahbar R, Fynn-Thompson F, Management of congenital tracheal stenosisPediatrics 2015 136(3):e660-69.10.1542/peds.2014-393126304826 [Google Scholar] [CrossRef] [PubMed]
[10]. Özer EA, Cumurcu S, Bayol Ü, Özdemir SA, Ilhan Ö, Sütçüoğlu S, Congenital complete tracheal ring in a neonate: A case reportTurk Patoloji Derg 2017 33(3):259-61.10.5146/tjpath.2014.01292 [Google Scholar] [CrossRef]
[11]. Dwivedi D, Dwivedi G, Gupta V, Kate S, Pediatric airway management in undiagnosed congenital subglottic stenosis patientsIndian Anaesth Forum 2020 21:70-73.https://doi.org/10.4103/TheIAForum.TheIAForum_63_1910.4103/TheIAForum.TheIAForum_63_19 [Google Scholar] [CrossRef]
[12]. Pawar DK, Doctor JR, Raveendra US, Ramesh S, Shetty SR, Divatia JV, All India Difficult Airway Association 2016 guidelines for management of unanticipated difficult tracheal intubation in PaediatricsIndian J Anaesth 2016 60:906-14.10.4103/0019-5049.19548328003692 [Google Scholar] [CrossRef] [PubMed]
[13]. Kundal R, Bharadwaj A, Dogra N, Jain P, Nanda S, Kundal VK, Anaesthetic management of unexpected subglottic stenosis in a neonate with tracheoesophageal fistulaJCR 2013 3:217-19.https://doi.org/10.17659/01.2013.005110.17659/01.2013.0051 [Google Scholar] [CrossRef]