Clinicolaboratory Profile and Outcome of Patients with Urosepsis at a Tertiary Care Centre in Southern India
Hagera Gulam Ahmed1, Gurajala Swathi2, Anukolu Reddy Ravishankar3, HRV Raj Kumar4, Lakshmi Vemu5
1 Postgraduate Student, Department of Microbiology, Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India.
2 Assistant Professor, Department of Microbiology, Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India.
3 Professor, Department of Microbiology, Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India.
4 Professor, Department of Microbiology, Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India.
5 Professor, Department of Microbiology, Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Lakshmi Vemu, Professor, Department of Microbiology, Kamineni Academy of Medical Sciences, LB Nagar, Hyderabad, Telangana, India.
E-mail: lakshmi57vemu@gmail.com
Introduction
Urosepsis is a serious, life-threatening consequence of a complicated Urinary Tract Infection (UTI). It is caused by bacterial infection of the urinary tract or prostate that spreads into the bloodstream. Since urosepsis is associated with a very high mortality rate (20-40%), an early diagnosis and identification of the causative bacteria is important so as to facilitate a prompt treatment with appropriate antibiotics. Nearly 50% cases of urosepsis are caused by the Gram Negative Bacterial (GNB) pathogen, Escherichia coli(E. coli).
Aim
To determine the bacteriological profile, antimicrobial susceptibility pattern, phenotypic resistance of the organisms associated with urosepsis and to correlate the levels of proinflammatory markers with the clinical outcome of the patient associated with urosepsis.
Materials and Methods
This was a prospective observational study including all patients with simultaneously positive urine and blood cultures, with identical bacterial isolate(s). The details of clinical presentation, antibiotic therapy and other relevant information such as C-Reactive Protein (CRP) and Procalcitonin (PCT) values were recorded and analysed using Microsoft office excel 2013.
Results
E. coli was the commonest isolate (43/53, 81.1%). Of the total 43 E. coli isolates, 4/43 (9.3%) were Extended Spectrum Beta Lactamase (ESBL) producers and 23/43 (53.49%) were Carbapenemase producers. Significant rise of the proinflammatory markers (PCT >10 ng/mL) and (CRP >100 mg/L) were associated with high mortality (49%). Out of the 53 patients, 43.4% (23/53) patients had more than one risk factor associated with severe sepsis and poor prognosis.
Conclusion
Early recognition of symptoms followed by accurate diagnosis and early goal directed therapy is essential to decrease morbidity and mortality from urosepsis.
Antibiotic susceptibility, Bacteriological profile, C-reactive protein, E.coli, Procalcitonin
Introduction
Urosepsis is defined as sepsis which is caused by infection of the urogenital tract and is a systemic response to infection. It is a life-threatening organ dysfunction caused due to a dysregulated host response to infection [1]. The signs and symptoms associated with Systemic Inflammatory Response Syndrome (SIRS) are now considered to be alerting symptoms which were initially considered to be “mandatory” for the diagnosis of sepsis [2,3]. “Sepsis” is a mosaic inflammatory response of the host to infection, often associated with a high mortality [4]. In about 30% of all septic patients, the infectious focus is localised in the urogenital tract [5]. The incidence of urosepsis increases with risk factors like age (≥65 years), diabetes mellitus, immune suppression (organ transplantation, chemotherapy, corticosteroid treatment, Acquired Immune Deficiency Syndrome (AIDS), nosocomial UTI and prior urological interventions [6,7].
Urosepsis must be detected at an early stage and promptly treated with appropriate antimicrobial agents to prevent complications [4]. The defining criteria for sepsis along with signs and symptoms of underlying cause of infection should be considered in the evaluation of sepsis. Urinalysis, urine culture and blood cultures must be performed in all patients with sepsis before antibiotic treatment is started [8]. Proinflammatory markers like PCT, CRP and Interleukin–6 (IL-6) also aid in the diagnosis of sepsis but the values should be interpreted in accordance with the cultures [9]. Outcome of the patients also depends on the infecting pathogen, early diagnosis and appropriate antibiotic therapy.
An early diagnosis and identification of the causative bacteria of urosepsis is important so as to facilitate a prompt treatment with appropriate antibiotics. In this study, the levels of proinflammatory markers in relation to mortality due to urosepsis have been evaluated. The present study was conducted to determine the bacteriological profile, antimicrobial susceptibility pattern and the resistance phenotype of the organism causing urosepsis.
Materials and Methods
A prospective observational study was conducted in the Microbiology Laboratory at Tertiary Care Hospital, for a period of two years from January 2018–December 2019. Institutional Ethical Clearance was obtained (KAMSRC/IEC/17/2017). Informed consent was obtained from the patients to use their clinical information for the study.
Urine specimen received from the patients, were inoculated by semi-quantitative method on chromogenic agar (CPSID, bioMerieux, France). The plates were incubated at 37°C for 24 hours. A growth of an isolate with more than 1 lac Colony Forming Units per milliliter (>105 CFU/mL) of urine was considered as significant bacteriuria [10]. Two sets FA plus (resin aerobic) and FN plus (resin anaerobic) of Bact/Alert blood cultures (bioMerieux, USA) were received from each patient. The bottles were loaded into BacT/Alert system and incubated at 37°C for five days. The samples flagged positive were unloaded from the system and subcultured on blood agar and chromogenic agar. The plates were incubated at 37°C for 24 hours. Any amount of growth of pathogenic bacteria on the plates was considered as significant [10].
All patients with simultaneously positive urine and blood cultures with an identical bacterial isolate were included in the study. Repeat isolates from the same patient were excluded from the study. The isolates were identified by VITEK 2 system (bioMerieux, France) using Identification cards for GNB (ID GN) (for GNBs) and Identification cards for GPC (ID GP) cards (for Gram Positive Cocci (GPCs) were used. Antimicrobial susceptibility testing was performed on the isolates on the VITEK2 system using the AST 628 (for GPCs) and AST 280 and 281 (for GNBs). The classes of antibiotics tested against GNB were Cephalosporins (3rd and 4th generation), Carbapenems, Polymyxins, Aminoglycosides, Quinolones and Beta lactam-Beta lactamase inhibitor combinations [11]. For GPCs, the antibiotic classes were Beta lactams, Aminoglycosides, Macrolides, Quinolones, Glycopeptides and Tetracyclines [11]. Multidrug resistance was documented in isolates showing resistance to more than three classes of antibiotics [12]. The details of clinical presentation, antibiotic therapy and other relevant information such as CRP (latex CRP Mono Reagent, Life labs, India) and PCT (Chemiluminescence Beckman Coulter, USA) values were recorded. The severity of sepsis was correlated with the levels of the proinflammatory markers, CRP (>100 mg/L) and PCT (>10 ng/mL). The levels of PCT were graded as mild or local bacterial infection (<0.5 ng/mL), SIRS (0.5-1.9 ng/mL), Sepsis (2-10 ng/mL) and severe sepsis (>10 ng/mL) [13].
Statistical Analysis
Percentages were calculated using Microsoft office excel 2013.
Results
Out of the total 4033 urine cultures obtained from suspected UTI patients during the study period, 1402 (34.76%) had significant bacteriuria. Out of 1402 patients, 53 (3.78%) patients also had positive blood cultures with the same organism(s) and hence fulfilled the definition of urosepsis. These 53 patients were included in the study for further analysis. The mean age of the patients was 63.92 years. [Table/Fig-1] shows age and sex distribution of the patients. Of these 53 patients, 37 (69.8%) patients were >60 years of age. The male to female distribution was 1:1.12. (25 Males and 28 Females). Common risk factors for developing urosepsis is shown in [Table/Fig-2]. Out of the 53 patients, 43.4% (23/53) had more than one of the risk factors associated with severe sepsis and poor prognosis.
Age and sex distribution table (N= 53).
| Age group (years) | Female | Percentage | Male | Percentage | Grand total | Percentage |
|---|
| 30-39 | 1 | 1.89 | 0 | 0 | 1 | 1.89 |
| 40-49 | 1 | 1.89 | 6 | 11.32 | 7 | 13.21 |
| 50-59 | 2 | 3.77 | 6 | 11.32 | 8 | 15.09 |
| 60-69 | 14 | 26.42 | 7 | 13.21 | 21 | 39.62 |
| 70-79 | 8 | 15.09 | 4 | 7.55 | 12 | 22.64 |
| >80 | 2 | 3.77 | 2 | 3.77 | 4 | 7.55 |
| Grand total | 28 | 52.83 | 25 | 47.17 | 53 | 100 |
Risk factors associated with urosepsis.
| Risk factor | No. of patients (N=53) | Percentage |
|---|
| Age >60 years | 37 | 69.8 |
| Diabetes | 34 | 64.2 |
| Urinary Catheter | 19 | 35.84 |
| Chronic Kidney Disease | 12 | 26.5 |
| Immunosupression | 4 | 7.55 |
| Prostatomegaly | 6 | 11.32 |
| Others* | 4 | 7.55 |
*Others- Recurrent UTI, Renal calculi, Cystoscopy
All 53 patients had a monomicrobial infection (51 GNB and 2 Enterococcus). [Table/Fig-3] shows the distribution of the isolates and [Table/Fig-4] shows the antibiotic susceptibility profile of the GNB. There was a 52.94% (27/51) multidrug resistance (Carbapenem resistance) among GNB isolates. Of the 51 GNB isolates, Enterobacteriaceae isolates were 49 (96.08%) and among these, E. coli was the commonest isolate (43/49, 87.75%). Of the total 43 E. coli isolates, 4/43 (9.3%) were only ESBL producers and 23/43 (53.49%) were ESBL and Carbapenemase producers. Of the total 6 K.pneumoniae isolates, 1/6 (16.67%) was only ESBL producer and 3/6 (50%) were ESBL and Carbapenemase producers.
Distribution of isolates.
| Name of the organism | Number | Percentage |
|---|
| E.coli | 43 | 81.14 |
| Klebsiella pneumoniae | 6 | 11.32 |
| Pseudomonas aeruginosa | 2 | 3.77 |
| Enterococcus spp | 2 | 3.77 |
| Total | 53 | 100 |
Antimicrobial susceptibility pattern of GNB isolates (N=51).
| Antibiotic | E.coli (N=43) | Klebsiella spp (N=6) | Pseudomonas aeruginosa (N=2) |
|---|
| No. | % Susceptible | No. | % Susceptible | No. | % Susceptible |
|---|
| Amoxicillin-clavulanic acid | 32 | 74.4 | 4 | 66.6 | NA | NA |
| Piperacillin-tazobactam | 32 | 74.4 | 4 | 66.6 | 0 | 0 |
| Cefotaxime | 16 | 37.20 | 2 | 33.3 | NA | NA |
| Ceftazidime | 16 | 37.20 | 2 | 33.3 | 0 | 0 |
| Cefepime | 16 | 37.20 | 2 | 33.3 | 0 | 0 |
| Cefaperazone-sulbactum | 32 | 74.4 | 4 | 66.6 | 0 | 0 |
| Ertapenem | 20 | 46.51 | 3 | 50 | NA | NA |
| Imipenem | 20 | 46.51 | 3 | 50 | 1 | 50 |
| Meropenem | 20 | 46.51 | 3 | 50 | 1 | 50 |
| Amikacin | 12 | 27.9 | 2 | 33.3 | 0 | 0 |
| Gentamicin | 12 | 27.9 | 2 | 33.3 | 0 | 0 |
| Ciprofloxacin | 6 | 4.65 | 0 | 0 | 0 | 0 |
| Levofloxacin | 6 | 4.65 | 0 | 0 | 0 | 0 |
| Colistin | 43 | 100 | 6 | 100 | 2 | 100 |
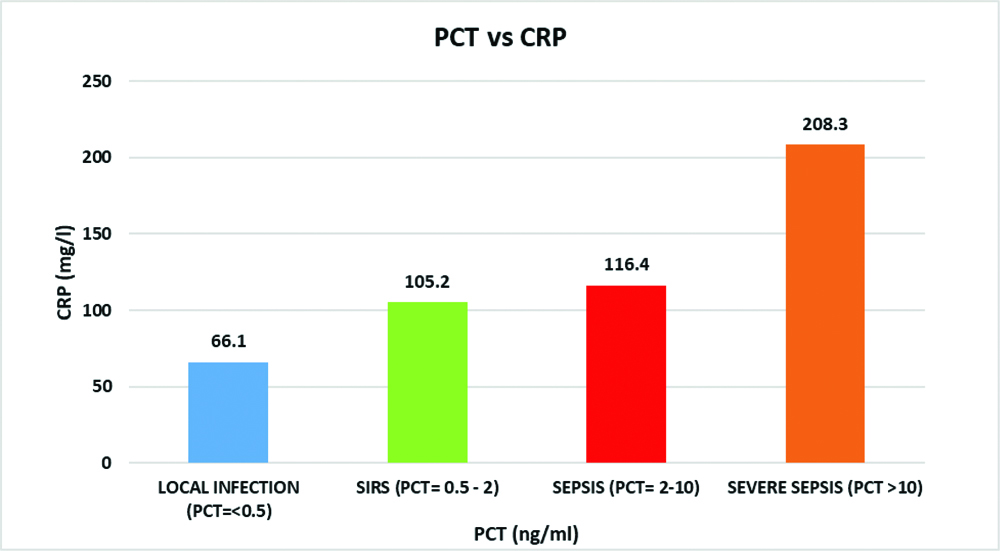
Out of the 53 patients, 75.5% (40/53) progressed to severe sepsis leading to septic shock and multi-organ failure and of which 26/40 (65%) had a fatal outcome {20/26 (77%) females and 6/26 (23%) males}. Severe sepsis and high mortality of 84.6% (22/26), were associated with resistant phenotypes (ESBL and Carbapenemases producers {E.coli (23) and Klebsiellapneumonia (3)}. Of the 2 Pseudomonas aeruginosa isolates, 1 isolate showed resistance to carbapenems. Both these patients with Pseudomonas aeruginosa infection had severe sepsis and a fatal outcome with significant levels of proinflammatory markers (PCT and CRP). In all, there were 2 Enterococcus isolates and both were resistant to Beta lactams and Macrolides but susceptible to vancomycin, teicoplanin and linezolid. Both these patients succumbed to the sepsis with significant levels of proinflammatory markers (PCT and CRP). In the present study, as shown in [Table/Fig-5], 22.6% (12/53) had PCT values 2-10 ng/mL (Sepsis) and 28/53 patients (52.8%) had a value of >10 ng/mL (Severe sepsis). A direct correlation between the PCT levels and poor clinical out-come of the patients in these two groups with severe sepsis was noted with a 65% mortality (26/40). A mean PCT of 17 ng/mL and a mean CRP of 194 mg/L were observed in all the 26 patients who succumbed to the urosepsis. Remainder of the PCT values were <0.5 ng/mL among 8 (15.1%) patients (Local infection) and 0.5-2 ng/mL among 5 (9.5%) patients (SIRS) [Table/Fig-5]. [Table/Fig-6] shows a correlation between the PCT and CRP values with the severity of sepsis. In case of severe sepsis with a PCT of >10 ng/mL, a proportionate increase of serum CRP was noted with an average of 208.3 mg/L. Similarly, in case of sepsis with PCT of 2-10 ng/mL, the mean CRP was 116.4 mg/L. In case of local infection and SIRS, there was no significant rise of CRP, which was 66.1 mg/L and 105.2 mg/L respectively.
Showing patient outcome and PCT range.
| PCT range | No. of patients (N=53) | No. of mortality (%) |
|---|
| <0.5 ng/mL | 8 | NIL |
| 0.5-1.9 ng/mL | 5 | NIL |
| 2-10 ng/mL | 12 | 3 (5.7%) |
| >10 ng/mL (Avg value=17.0) | 28 | 23 (43.4%) |
Discussion
Urosepsis resulting from complicated UTI in patients with associated underlying risk factors increases morbidity and mortality. The condition can be usually identified early in its course with a basic diagnostic evaluation comprising of risk factor analysis, urine and blood cultures and proinflammatory marker levels for sepsis. Successful management of patients with urosepsis depends on isolating the causative agent and instituting early and specific antibiotic therapy, along with eliminating the infectious foci in the urinary tract [14].
Age of the patients is one of the risk factors. In the present study, 37/53 (69.8%) of the patients were ≥60 years of age with a mean age of 63.92 years, and a mortality of 26/37 (70.3%). Other studies observed a mean age range of 60–83.6 years [15-17], with a mortality rate of 33% [16]. Underlying co-morbid conditions, especially diabetes mellitus has been shown to be associated with development of pyelonephritis and further progressing to urosepsis [15,18]. In the present study, Type 2 DM was a major risk factor among 34/53 (64%) patients. Of the 34 diabetic patients, 32/34 (94.11%) were elderly patients (>60 years), of which 23 patients (71.88%) died of urosepsis.
In the present study, all the 53 cases were of monomicrobial infections with GNB being the predominant isolates 51/53 (96.2%). E. coli was the commonest causative organism, 43/53 (81.1%). The isolation rate of E. coli from other studies were 46.1% [16], 69% [19], 75% [15] and 79% [17]. Multidrug resistant urosepsis is being increasingly reported in various studies [15,19]. This can be attributed to recurrent infections, repeated hospitalisations of the patients and indiscriminate use of antibiotics [20].
PCT is a valuable proinflammatory marker in the diagnosis of bacterial sepsis, determining the severity of sepsis and the duration of antibiotic administration [21]. During an infection, PCT is released up to a thousand fold increase from nearly all tissues and cell types in the host in response to cytokines and bacterial products [22]. Patients with urosepsis and shock also exhibit a significantly higher CRP as compared with urosepsis patients without shock [22-24]. A direct correlation between the levels of PCT and CRP in sepsis and septic shock and mortality was also documented by Brunkhorst FM et al., [22].
In the present study, 40/53 (75.5%) patients progressed to severe sepsis leading to septic shock and multi-organ failure. A mean PCT of 17 ng/mL and a mean CRP of 194 mg/L were observed in all the 26/53 patients who succumbed to the urosepsis, in the present study. The values were low among the survivors, as was also documented by another Indian study (PCT of 12 ng/mL) [23]. and the mortality rate of the patients associated with these resistant isolates was 22/26 (84.6%) [23,24].
There is high mortality rate associated with Multidrug resistant organisms (MDROs) for which a combination therapy of carbapenem plus a Polymyxin B would be effective. Similar studies done on MDROs causing blood stream infections showed a 2-3 fold increase in mortality rate and treatment failure [25,26]. To decrease the mortality rate among MDROs various studies [27,28], have shown effective therapy in such cases to be a combination therapy with Carbapenem plus a Polymyxin B compared to monotherapy.
Limitation(s)
This study had certain limitations. There was no control group, (UTI with negative blood cultures) to compare the risk factors and values of the biomarkers which can further improve the diagnosis for the clinicians in order to treat patients on risk.
Conclusion(s)
Early recognition of symptoms followed by accurate diagnosis and early goal directed therapy is essential to decrease morbidity and mortality from urosepsis. Patients with existing co-morbid conditions must be educated on the possible risk of UTI leading to urosepsis. They must be advised to seek early intervention in order to avoid poor outcome. A multidisciplinary team including microbiologists, surgeons, infectious disease physicians and radiologists with a coordinated effort is essential for a favourable outcome.
*Others- Recurrent UTI, Renal calculi, Cystoscopy
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 25, 2020
Manual Googling: Dec 07, 2020
iThenticate Software: Dec 19, 2020 (13%)
[1]. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)JAMA 2016 315(8):801-10.10.1001/jama.2016.028726903338 [Google Scholar] [CrossRef] [PubMed]
[2]. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care MedicineChest 1992 101(6):1644-55.10.1378/chest.101.6.16441303622 [Google Scholar] [CrossRef] [PubMed]
[3]. Bone RC, Sprung CL, Sibbald WJ, Definitions for sepsis and organ failureCrit Care Med 1992 20:724-26.10.1097/00003246-199206000-000021597021 [Google Scholar] [CrossRef] [PubMed]
[4]. Emanuel R, Bryant N, Suzanne H, Julie R, Alexandria M, Benhard K, Early goal-directed therapy in the treatment of severe sepsis and septic shockN Engl J Med 2001 345:1368-77.10.1056/NEJMoa01030711794169 [Google Scholar] [CrossRef] [PubMed]
[5]. Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Outcomes of the surviving sepsis campaign in intensive care units in the USA and Europe: A prospective cohort studyLancet Infect Dis 2012 12(12):919-24.10.1016/S1473-3099(12)70239-6 [Google Scholar] [CrossRef]
[6]. Martin GS, Mannino DM, Moss M, The effect of age on the development and outcome of adult sepsisCrit Care Med 2006 34:15-21.10.1097/01.CCM.0000194535.82812.BA16374151 [Google Scholar] [CrossRef] [PubMed]
[7]. Bjerklund Johansen TE, Cek M, Naber K, Stratchounski L, Svendsen MV, Tenke P, PEP and PEAP study investigators; European Society of Infections in Urology. Prevalence of hospital-acquired urinary tract infections in urology departmentsEur Urol 2007 51(4):1100-11.10.1016/j.eururo.2006.08.01217049419 [Google Scholar] [CrossRef] [PubMed]
[8]. ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, A randomized trial of protocol-based care for early septic shockN Engl J Med 2014 370(18):1683-93.10.1056/NEJMoa140160224635773 [Google Scholar] [CrossRef] [PubMed]
[9]. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J, Serum procalcitonin and C- reactive protein levels as markers of bacterial infection: A systematic review and meta-analysisClin Infect Dis 2004 39:206-17.10.1086/42199715307030 [Google Scholar] [CrossRef] [PubMed]
[10]. Collee JG, Fraser AG, Marmion BP, Simmons A, Mackie and McCartney, Practical Medical Microbiology 1996 14th EditionChurchill Livingstone [Google Scholar]
[11]. CLSIPerformance standards for antimicrobial susceptibility testing 2019 29th edition. CLSI supplement M101Wayne, PAClinical and Laboratory Standards Institute [Google Scholar]
[12]. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistanceClin Microbiol Infect 2012 18(3):268-81.10.1111/j.1469-0691.2011.03570.x21793988 [Google Scholar] [CrossRef] [PubMed]
[13]. Sharma S, Duggal N, Role of procalcitonin, IL-6 and C-reactive protein in suspected cases of sepsisIndian J Pathol Microbiol 2019 62:578-81.10.4103/IJPM.IJPM_762_1831611443 [Google Scholar] [CrossRef] [PubMed]
[14]. Sugimoto K, Adomi S, Koike H, Esa A, Procalcitonin as an indicator of urosepsisRes Rep Urol 2013 5:77-80.10.2147/RRU.S4271124400237 [Google Scholar] [CrossRef] [PubMed]
[15]. Bijou MR, Bhat KS, Kanungo R, Characteristics of blood stream isolates in urosepsis from a tertiary care hospitalInt J Curr Microbiol App Sci 2016 5(10):424-31.10.20546/ijcmas.2016.510.048 [Google Scholar] [CrossRef]
[16]. Tal S, Guller V, Levi S, Bardenstein R, Berger D, Gurevich I, Profile and prognosis of febrile elderly patients with bacteremic urinary tract infectionJ Infect 2005 50:296-305.10.1016/j.jinf.2004.04.00415845427 [Google Scholar] [CrossRef] [PubMed]
[17]. van Nieuwkoop C, Bonten TN, van’t Wout JW, Kuijper EJ, Groeneveld GH, Becker MJ, Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: A prospective observational studyCrit Care 2010 14(6):R20610.1186/cc932821083886 [Google Scholar] [CrossRef] [PubMed]
[18]. Garg V, Bose A, Jindal J, Goyal A, Comparison of clinical presentation and risk factors in diabetic and non-diabetic females with urinary tract infection assessed as per the European Association of Urology ClassificationJ Clin Diagn Res 2015 9(6):12-14.10.7860/JCDR/2015/14177.602926266160 [Google Scholar] [CrossRef] [PubMed]
[19]. Chen LF, Chiu CT, Lo JY, Tsai SY, Weng LS, Anderson DJ, Clinical characteristics and antimicrobial susceptibility pattern of hospitalised patients with community acquired urinary tract infections at a regional hospital in TaiwanHealthc Infect 2013 19(1):20-25.10.1071/HI1303325580164 [Google Scholar] [CrossRef] [PubMed]
[20]. Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO, Extended-spectrum β- lactamase-producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on outcomesClin Infect Dis 2001 32(8):1162-71.10.1086/31975711283805 [Google Scholar] [CrossRef] [PubMed]
[21]. Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L, Procalcitonin and C reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunctionCrit Care 2004 8:234-42.10.1186/cc287715312223 [Google Scholar] [CrossRef] [PubMed]
[22]. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R, Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shockIntensive Care Medicine 2000 26(0):S148-52.10.1007/s001340051134 [Google Scholar] [CrossRef]
[23]. Nanda SK, Dinakaran A, Bhat KS, Ravichandran Kanungo R, Diagnostic and prognostic role of procalcitonin in sepsis in a tertiary care hospitalBiomedical Research 2015 27(1):79-83. [Google Scholar]
[24]. Ruiz-Alvarez MJ, García-Valdecasas S, De Pablo R, Diagnostic efficacy and prognostic value of serum procalcitonin concentration in patients with suspected sepsisJ Intensive Care Med 2009 24:63-71.10.1177/088506660832709519054806 [Google Scholar] [CrossRef] [PubMed]
[25]. Gandra S, Tseng KK, Arora A, Bhowmik B, Robinson ML, Panigrahi B, The mortality burden of multidrug-resistant pathogens in India: A retrospective, observational studyClin Infect Dis 2019 69(4):563-70.10.1093/cid/ciy95530407501 [Google Scholar] [CrossRef] [PubMed]
[26]. Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapyClin Infect Dis 2012 55(7):943-50.10.1093/cid/cis58822752516 [Google Scholar] [CrossRef] [PubMed]
[27]. Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, Combination therapy for carbapenem-resistant gram-negative bacteriaJ Antimicrob Chemother 2014 69(9):2305-09.10.1093/jac/dku16824872346 [Google Scholar] [CrossRef] [PubMed]
[28]. Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenemsAntimicrob Agents Chemother 2013 57(10):5104-11.10.1128/AAC.01230-1323917322 [Google Scholar] [CrossRef] [PubMed]