Susceptibility of Clinical Isolates of Staphylococcus aureus to Ceftaroline
Harsha Sreedharan1, KB Asha Pai2
1 MBBS Student, KS Hegde Medical Academy, NITTE (Deemed to be University), Mangalore, Karnataka, India.
2 Associate Professor, Department of Microbiology, KS Hegde Medical Academy, NITTE (Deemed to be University), Mangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. KB Asha Pai, Associate Professor, Department of Microbiology, KS Hegde Medical Academy, NITTE (Deemed to be University), Mangalore-575018, Karnataka, India.
E-mail: ashamkamath@gmail.com
Introduction
Methicillin-Resistant Staphylococcus aureus (MRSA) infection is a major global healthcare problem, the prevalence of which varies from 25-50% in India. It is known to cause Skin and Soft tissue Infections (SSI), endovascular infections, endocarditis, pneumonia, septic arthritis, osteomyelitis, and sepsis. Vancomycin is the drug of choice for treating severe MRSA infections. Ceftaroline, a fifth-generation cephalosporin has been approved by the United States Food and Drug Administration (US FDA) for treating acute bacterial SSI caused by susceptible micro-organisms including MRSA, Community acquired respiratory tract infection, MRSA bacteremia and endocarditis.
Aim
To assess the susceptibility of clinical isolates of S. aureus to ceftaroline, in a Tertiary Care Hospital.
Materials and Methods
This prospective study was conducted in the Department of Microbiology of a Tertiary Care Hospital over a period of two months from June 2019 to July 2019. S.aureus isolates from various clinical samples were screened for methicillin resistance by disc diffusion method using cefoxitin disc and ceftaroline susceptibility of these isolates was assessed by E-strip method. The isolates were classified as ceftaroline susceptible, Susceptibility Dose Dependent (SDD) and ceftaroline resistant respectively as per CLSI guidelines. A descriptive analysis of the data was done and the results were presented as frequencies and percentages.
Results
All the S.aureus isolates were found to be susceptible to ceftaroline. Methicillin Sensitive Staphylococcus aureus (MSSA) isolates had lower Minimum Inhibitory Concentration (MIC) when compared to MRSA. The highest MIC among MRSA was 0.5 μg/mL.
Conclusion
Ceftaroline can be considered as an effective alternative for treatment of infections caused by MRSA.
Ceftaroline susceptibility, Methicillin resistant Staphylococcus aureus, Minimum inhibitory concentration
Introduction
The MRSA infection is a major global healthcare problem, the prevalence of which varies from 25-50% in India [1]. It is known to cause Skin and Soft tissue Infection (SSI), endovascular infections, endocarditis, pneumonia, septic arthritis, osteomyelitis, and sepsis [2]. Vancomycin is the drug of choice for treating severe MRSA infections. However, the use of vancomycin has been associated with several limitations which include poor penetration of the drug into the tissues, narrow therapeutic index, slow bactericidal activity, difficulty in achieving pharmacokinetic/pharmacodynamic targets and potential side effects like nephrotoxicity and ototoxicity [3,4]. Also, a meta-analysis has reported treatment failures with vancomycin therapy in critically ill patients which may be attributed to suboptimal therapeutic levels or high MIC values [5]. Alternative drugs like linezolid, daptomycin are being used increasingly for treatment of MRSA infections [6].
Ceftaroline, a fifth-generation cephalosporin has been approved by the US FDA for treating acute bacterial SSI caused by susceptible micro-organisms including MRSA, community acquired respiratory tract infection, MRSA bacteremia and endocarditis [7]. This antimicrobial inhibits cell wall synthesis by binding to Penicillin Binding Proteins (PBP) 1, 2, 3 and PBP 2a for MRSA [8]. Clinical trials have shown that ceftaroline is well tolerated by patients [9]. Also, it has been shown to be as effective as vancomycin, daptomycin and linezolid in eradicating MRSA [9,10]. Resistance to ceftaroline is not very common. Several studies have reported decreased susceptibility of MRSA to ceftaroline in sporadic cases [11,12]. The resistance may be due to the mutation within PBP 2a protein, in particular, outside the Penicillin- Binding Domain (nPBD) [7].
In India, there are very few studies undertaken to evaluate the susceptibility of S.aureus to ceftaroline and there is a limited data about the susceptibility pattern of S.aureus to ceftaroline [13-15]. Therefore, this study was conducted to screen S.aureus isolates obtained from various clinical samples for methicillin resistance and assess their susceptibility to ceftaroline, in a Tertiary Care Hospital.
Materials and Methods
This prospective study was conducted in the Department of Microbiology over a period of two months from June 2019 to July 2019 as part of ICMR-STS 2019 (Reference ID.2019-02280) Clearance was obtained from the Institutional Ethics Committee (INST.EC/EC/057/2019-20).
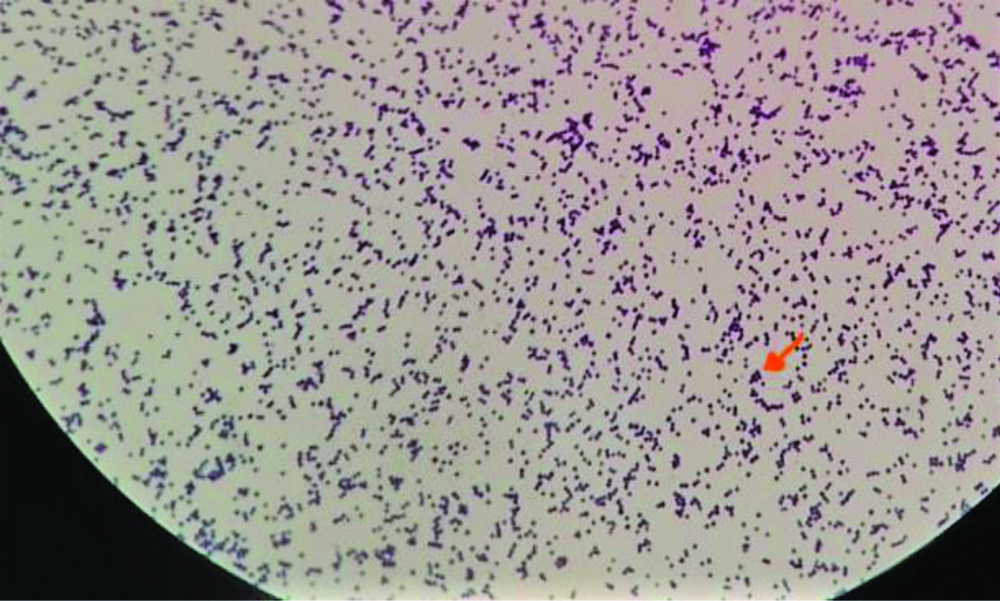
Fifty non-duplicate S. aureus strains isolated from various clinical samples were included in the study. The strains were streaked on nutrient agar plates after thawing the vials. A smear was prepared from an isolated colony and stained with Gram’s stain [Table/Fig-1]. Biochemical tests like catalase test and tube coagulase test were done to reconfirm the identity of the strain. Gram positive cocci which were catalase positive and tube coagulase positive [Table/Fig-2] were identified as Staphylococcus aureus.
Gram positive cocci in singles, pairs and clusters.
(magnification- X1000)
Tube coagulase test. The arrow showing positive tube coagulase yielded by the test isolate.
NC: Negative control, PC: Positive control; T: Test isolate

Screening for MRSA: Screening for methicillin resistance was done by modified Kirby Bauer disc diffusion method using cefoxitin (30 μg) discs [16]. Inoculum for lawn culture was prepared by direct colony suspension method. Three to five isolated colonies of S. aureus were suspended in 5 mL of peptone water and the turbidity of the test suspension was standardised to match 0.5 McFarland standard. A lawn culture of the test organism was made on Mueller Hinton agar plates according to standard protocols. Cefoxitin disc (30 μg) was placed on the lawn culture and the plates were incubated at 35°C for 16-18 hours. The diameter of the zone of inhibition was measured using a ruler. A zone size of ≥22 mm was interpreted as methicillin sensitive and ≤21 mm was interpreted as methicillin resistant as per Clinical and Laboratory Standards Institute (CLSI) guidelines. S. aureus American Type Culture Collection (ATCC) 25923 and S. aureus ATCC 43300 were used as controls [16].
Ceftaroline susceptibility: Testing for ceftaroline susceptibility was done by E-strip method. The ceftaroline E strips 0.002-32 μg/mL was obtained from Biomerieux, France. Inoculum preparation and lawn culture of the test organism was done as detailed for disc diffusion method. The E-strips were placed on the lawn culture and the plates were incubated at 37°C for 18-24 hours. MIC’s were read where the ellipse intersects the MIC scale. Since E-strip has continuous gradient, MIC values “in-between” two-fold dilutions can be obtained. These values were rounded up to next two-fold dilution before categorisation. MIC ≤1 μg/mL, 2-4 μg/mL and ≥8 μg/mL were interpreted as ceftaroline susceptible, SDD and ceftaroline resistant respectively as per CLSI guidelines. S. aureus ATCC 29213 was used as control [16].
Statistical Analysis
Descriptive analysis of the distribution of sample, age, gender, and antimicrobial susceptibility data were done, and the results obtained were presented as frequencies and percentages.
Results
Among the 50 S.aureus isolates 28 (56%) were isolated from male patients and 22 (44%) from females. The age range of patients from whom the S.aureus was isolated was 2-73 years, mean age being 41 years.
Of the 50 non-duplicate S. aureus isolates, 47 (94%) were isolated from pus, two (4%) from blood and one (2%) from endotracheal aspirate.
Among the 50 isolates tested 28 (56%) were methicillin resistant and 22 (44%) were methicillin sensitive. The age and gender distribution for MRSA and MSSA are shown in [Table/Fig-3].
Age and gender distribution for MRSA and MSSA.
| Age group (in years) | MRSA | MSSA |
|---|
| No. of males | No. of females | No. of males | No. of females |
|---|
| 0-20 | 3 | 0 | 2 | 1 |
| 21-40 | 3 | 8 | 3 | 2 |
| 41-60 | 5 | 4 | 8 | 4 |
| 61-80 | 2 | 3 | 2 | 0 |
| Total | 13 | 15 | 15 | 7 |
All the S. aureus isolates were found to be susceptible to ceftaroline with their MIC’s ranging from 0.064 to 0.5 μg/mL. Frequency distribution of ceftaroline MIC’s of MSSA and MRSA isolates are shown in [Table/Fig-4]. It was also observed that the MSSA isolates had lower MIC’s when compared to MRSA. Majority (90%) of MSSA isolated had an MIC of ≤0.25 μg/mL in comparison to 13 (46.43%) of MRSA isolates which had an MIC of ≤0.25 μg/mL. A MIC of 0.5 μg/mL was observed among 15 (53.57%) of the MRSA isolates which included 14 isolates having MIC of 0.38 μg/mL rounded-up to 0.5 μg/mL.
Frequency distribution of ceftaroline MIC’s among MSSA and MRSA isolate.
| Ceftaroline MIC (μg/mL) | No. of MSSA isolates (%) | No. of MRSA isolates (%) |
|---|
| 0.064 | 3 (13.64) | 0 |
| 0.125 | 11 (50) | 0 |
| 0.25 | 6 (27.27) | 13 (46.43) |
| 0.5 | 2 (9.09) | 15 (53.57) |
| Total | 22 | 28 |
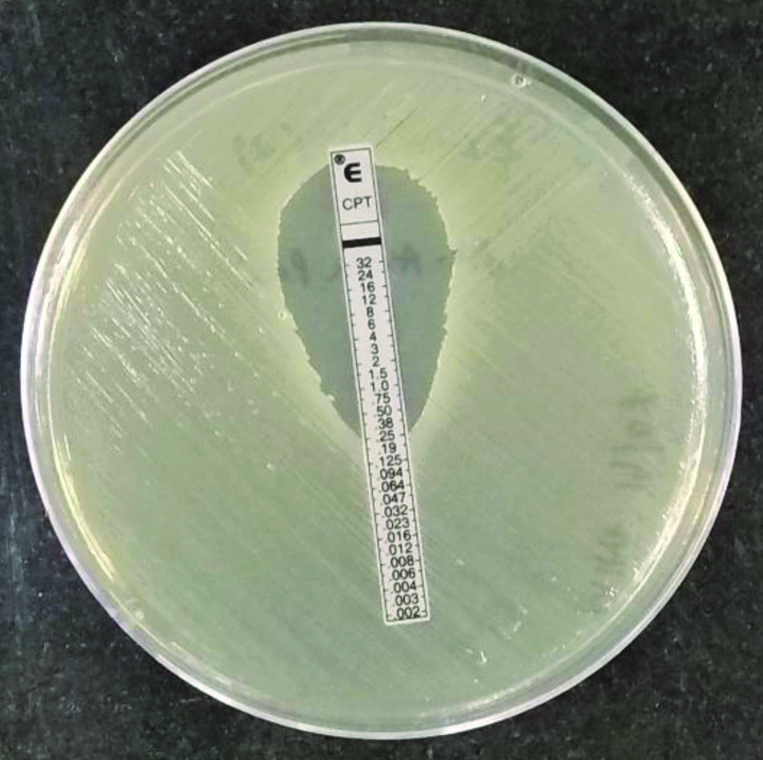
A MRSA isolate with ceftaroline MIC of 0.25 μg/mL is shown in [Table/Fig-5].
MRSA isolate with ceftaroline MIC of 0.25 μg/mL.
Discussion
Staphylococcus aureus is an important cause of hospital as well as community acquired infections. In the era of increasing antimicrobial resistance, treating the infections caused by MRSA is posing a real challenge to the clinicians. Emergence of multi-drug resistant MRSA isolates further complicates the treatment of infections caused by these organisms [13]. Ceftaroline fosamil has been approved as an alternative for a severe MRSA infection [8].
In this study, all the 50 clinical isolates of S. aureus, inclusive of 22 MSSA and 28 MRSA strains were found to be susceptible to ceftaroline. This corelates with the findings of an Indian study, where all the 50 MRSA isolates isolated from various clinical samples were found to be sensitive to ceftaroline [13]. Similar results were also observed in a multi-centre study from Spain, where the all S. aureus isolates tested were inhibited by ceftaroline with a MIC of ≤1 μg/mL [17]. The antimicrobial resistance surveillance program, Assessing Worldwide Antimicrobial Resistance and Evaluation (AWARE), which evaluated the trends in S. aureus susceptibility rates to ceftaroline, reported a 100% susceptibility to ceftaroline among MSSA. However, the study found that the susceptibility of MRSA to ceftaroline decreased marginally from 99.4% in 2010 to 98.6% in 2016 [18].
This study found that the MSSA isolates had much lower ceftaroline MIC’s when compared to MRSA isolates. Majority (90.9%) of MSSA isolates had an MIC ≤0.25 μg/mL. This correlates with the findings from a multi-centric study from India [14]. A multi-centric study from Latin American countries, as part of AWARE surveillance program, also reported a similar finding with 98.3% of MSSA isolates having a ceftaroline MIC of ≤0.25 μg/mL [19].
All the MRSA isolates in this study were found to be susceptible to ceftaroline with 0.5 μg/mL being the highest ceftaroline MIC detected. This is in contrast to other studies from India which have reported a higher ceftaroline MIC. Further details are shown in [Table/Fig-6] [14,15].
Comparison of ceftaroline MIC of MRSA strains in India [14,15].
| Authors | n | No. of strains of MRSA (%) inhibited at ceftaroline MIC (μg/mL) of: |
|---|
| | ≤1 | 2-4 | 8 |
| Bakthavatchalam YD et al., [14] | n=86 | 73 (84.88) | 13 (15.12) | Nil |
| Gaikwad V et al., [15] | n=30 | 28 (93.33) | 2 (6.67) | Nil |
| Present study | n=28 | 28 (100) | Nil | Nil |
MIC: Minimum inhibitory concentration; MRSA: Methicillin resistant staphylococcus aureus
One such study showed that 6% and 2% of tested S. aureus had a ceftaroline MIC of 2 μg/mL and 4 μg/mL, respectively [14], which according to the old Clinical and Laboratory Standards Institute (CLSI) guidelines were interpreted as intermediate and resistant respectively. If the current CLSI guidelines are applied, these isolates will be classified as SDD and not as resistant [14,16]. The authors of another study from Maharashtra, India reported that 93.33% MRSA isolates were susceptible to ceftaroline with the MIC being 0.75 μg/mL and concluded that it can be considered an effective alternative treatment, while vancomycin and linezolid can be kept as reserve drug [15]. A multicentric study across seven provinces in Turkey found that 94.3% of tested MRSA isolates were inhibited by ceftaroline (MIC≤1 μg/mL) [20]. In a study conducted in the US hospitals from 2008-2011, the authors found that all daptomycin non-susceptible Staphylococci isolates, 85.7% and 91.9% of linezolid-resistant S.aureus isolates and S.aureus isolates with a vancomycin MIC of ≥2 μg/mL respectively were susceptible to ceftaroline. The authors concluded that ceftaroline may be considered as a valuable treatment option for infections caused by multidrug resistant S. aureus [21].
The recommended dosage of ceftaroline is 600 mg administered every 12 hours by intravenous (IV) infusion over 60 minutes in patient’s ≥18 years of age [8]. Apart from the clinical efficacy, it is important to consider the adverse effects as well while prescribing any drug. The most common adverse effects with ceftaroline includes nausea, vomiting and diarrhoea. The incidence of which is 3-5% which was comparable with vancomycin/aztreonam with a dosing regimen of vancomycin 1g every 12 hours plus aztreonam 1g every eighth hourly [9]. Clinical trials have also shown that ceftaroline is as effective as ceftriaxone, and combination of vancomycin/aztreonam for the treatment of community-acquired pneumonia and complicated SSI, respectively [22].
Limitation(s)
This study has evaluated a small number of isolates, as it was an ICMR-STS project with a limited study duration of two months. Prospective studies with larger sample size are warranted to support or verify the findings. Secondly, the present study has not evaluated resistance of MRSA isolates to other antibiotics like vancomycin, linezolid, teicoplanin which may be considered as a limitation of this study.
Conclusion(s)
Taking into consideration, the high susceptibility rates and comparable or better tolerance of patients to ceftaroline, when compared to vancomycin, for the treatment of infections caused by MRSA isolates, ceftaroline can be considered as an effective alternative for treatment of infections caused by MRSA. But like most drugs, ceftaroline might become ineffective, if misused. With the increasing resistance to antibiotics and very few newer antibiotics in the pipeline, it is high time to stop misusing the antibiotics in order to help in combating the development of further resistance and prevent going back to the pre-antibiotic era.
MIC: Minimum inhibitory concentration; MRSA: Methicillin resistant staphylococcus aureus
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 01, 2020
Manual Googling: Nov 06, 2020
iThenticate Software: Dec 19, 2020 (18%)
[1]. Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, IndiaMethicillin Resistant Staphylococcus aureus (MRSA) in India: Prevalence and susceptibility patternIndian J Med Res 2013 137:363-69. [Google Scholar]
[2]. Al-Hamad AM, Alfaraj AA, Altowaileb JA, Al-Shamlan SM, Leskafi H, Alsubeikhy FA, Incidence and antibiotic susceptibility of MRSA infections in a Saudi Arabian Hospital: A 10-year surveillance studyJ Infect Dev Ctries 2018 12(6):454-67.10.3855/jidc.977831940297 [Google Scholar] [CrossRef] [PubMed]
[3]. Pletz MW, Burkhardt O, Welte T, Nosocomial methicillin -resistant Staphylococcus aureus (MRSA) pneumonia: Linezolid or vancomycin? - Comparison of pharmacological and clinical efficacyEur J Med Res 2010 15:507-13.10.1186/2047-783X-15-12-50721163725 [Google Scholar] [CrossRef] [PubMed]
[4]. Cosimi RA, Beik N, Kubiak DW, Johnson JA, Ceftaroline for severe Methicillin-Resistant Staphylococcus aureus infections: A systematic reviewOpen forum infectious diseases 2017 4(2):ofx08410.1093/ofid/ofx08428702467 [Google Scholar] [CrossRef] [PubMed]
[5]. Jacob JT, Diaz Granados CA, High vancomycin minimum inhibitory concentration and clinical outcomes in adults with methicillin-resistant Staphylococcus aureus infections: A meta-analysisInt J Infect Dis 2013 17:e93-100.doi: 10.1016/j.ijid.2012.08.005 [Google Scholar]
[6]. Micek ST, Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus InfectionsCID 2007 45:S184-90.10.1086/51947117712745 [Google Scholar] [CrossRef] [PubMed]
[7]. Slusarczyk R, Bielejewska A, Bociek A, Bociek M, Resistance to ceftaroline- 2018 reviewEuropean Journal of Biological Research 2018 8(3):112-20. [Google Scholar]
[8]. Laudano JB, Ceftaroline fosamil: A new broad-spectrum cephalosporinJ Antimicrob Chemother 2011 66:iii11-iii18.10.1093/jac/dkr09521482565 [Google Scholar] [CrossRef] [PubMed]
[9]. Lan SH, Chang SP, Lai CC, Lu LC, Chao CM, Ceftaroline efficacy and safety in treatment of complicated skin and soft tissue infection: A systemic review and meta-analysis of randomized controlled trialsJ Clin Med 2019 8(6):77610.3390/jcm806077631159264 [Google Scholar] [CrossRef] [PubMed]
[10]. Zasowski EJ, Trinh TD, Claeys KC, Casapao AM, Sabagha N, Lagnf AM, Multicenter observational study of ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bloodstream infectionsAntimicrob Agents Chemother 2017 61(2):e02015-16.10.1128/AAC.02015-16 [Google Scholar] [CrossRef]
[11]. Kelley WL, Jousselin A, Barras C, Lelong E, Renzoni A, Missense mutations in PBP2A Affecting ceftaroline susceptibility detected in epidemic hospital-acquired methicillin-resistant Staphylococcus aureus clonotypes ST228 and ST247 in Western Switzerland archived since 1998Antimicrob Agents Chemother 2015 59(4):1922-30.10.1128/AAC.04068-1425583724 [Google Scholar] [CrossRef] [PubMed]
[12]. Sader HS, Flamm RK, Jones RN, Antimicrobial activity of ceftaroline and comparator agents tested against bacterial isolates causing skin and soft tissue infections and community-acquired respiratory tract infections isolated from the Asia-Pacific region and South AfricaDiagn Microbiol Infect Dis 2010 76(1):61-68.10.1016/j.diagmicrobio.2013.01.005 [Google Scholar] [CrossRef]
[13]. Basireddy S, Singh M, Ali S, Kabra V, In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus isolatesIndian J Med Microbiol 2015 33:464-65.10.4103/0255-0857.15861226068368 [Google Scholar] [CrossRef] [PubMed]
[14]. Bakthavatchalam YD, Prasagam AK, Anandan S, Joshi S, Chaudhuri BN, Chitnis DS, Comparative in-vitro activity of Ceftaroline against Staphylococcus aureus isolates from IndiaJ Infect Dev Ctries 2016 10(3):109-12.10.3855/jidc.719626829546 [Google Scholar] [CrossRef] [PubMed]
[15]. Gaikwad V, Gohel T, Panickar S, Chincholkar V, Mangalkar S, In vitro activity of ceftaroline: A novel antibiotic against methicillin-resistant Staphylococcus aureusIndian J Pathol Microbiol 2016 59(4):496-98.10.4103/0377-4929.19179827721280 [Google Scholar] [CrossRef] [PubMed]
[16]. CLSIPerformance Standards for Antimicrobial susceptibility testing. 29th ed. CLSI supplement M100 2019 Wayne PAClinical and laboratory standards institute [Google Scholar]
[17]. Tenorio-Abreu A, Gil Tomás J, Bratos Pérez MÇ, de la Iglesia Salgado A, Borrás Máñez M, de Lejarazu O, In vitro activity of ceftaroline against Spanish isolates of Staphylococcus aureus: A multicenter studyEnfermedades Infecc Microbiol Clínica 2015 33:101-04.10.1016/j.eimc.2014.02.00925091384 [Google Scholar] [CrossRef] [PubMed]
[18]. Sader HS, Mendes RE, Streit JM, Flamm RK, Antimicrobial susceptibility trends among Staphylococcus aureus isolates from U.S. hospitals: Results from 7 years of the ceftaroline (AWARE) surveillance program, 2010 to 2016Antimicrob Agents Chemother 2017 61:e01043-1710.1128/AAC.01043-17 [Google Scholar] [CrossRef]
[19]. Biedenbach DJ, Hoban DJ, Reiszner E, Lahiri SD, Alm RA, Sahm DF, Invitro activity of ceftaroline against Staphylococcus aureus isolates collected in 2012 from Latin American countries as part of the AWARE surveillance programAntimicrob Agents Chemother 2015 59(12):7873-77.10.1128/AAC.01833-1526416860 [Google Scholar] [CrossRef] [PubMed]
[20]. Mengeloglu FZ, Taş T, Koçoglu E, Copur Çiçek A, Yanık K, Güneş H, In vitro activity of ceftaroline to MRSA isolates: A multicenter studyMikrobiyol Bul 2013 47:677-83.10.5578/mb.547924237436 [Google Scholar] [CrossRef] [PubMed]
[21]. Sader HS, Flamm RK, Jones RN, Antimicrobial activity of ceftaroline tested against Staphylococci with reduced susceptibility to linezolid, daptomycin, or vancomycin from U.S. hospitals, 2008 to 2011Antimicrob Agents Chemother 2013 57:3178-81.10.1128/AAC.00484-1323629712 [Google Scholar] [CrossRef] [PubMed]
[22]. Henry P, Mei HC, Horatio BF, Ceftaroline fosamil: A cephalosporin with activity against methicillin-resistant Staphylococcus aureusClinical Therapeutics 2012 34(4):743-65.10.1016/j.clinthera.2012.02.02522444785 [Google Scholar] [CrossRef] [PubMed]