Introduction
The cellular phones/mobile phones have emerged as the fastest growing man-made phenomenon ever discovered in the history. Controversies still exist among the scientific community regarding the ill-effects of Radiofrequency Radiation (RFR) exposure from cell phones on biological tissues. The present study will provide an insight into the basic mechanisms by which RF fields interact with developing brain in an embryo.
Aim
To assess the possible Deoxyribonucleic Acid (DNA) damage in developing brain of chick embryo following chronic exposure to Ultra-High Frequency/Radiofrequency Radiation (UHF/RFR) emitted from 2G and 3G cell phone.
Materials and Methods
Fertilised hen eggs were divided into three groups. Experimental Group A (exposed to 2G radiation, 24 eggs), Experimental Group B (exposed to 3G radiation, 24 eggs) and Group C sham exposed control group (24 eggs). After the completion of scheduled duration of exposure (72 minutes per day), the chick embryos were sacrificed from 9th-12th day and the brains were dissected out. The chick embryo brains were then subjected to alkaline comet assay technique to assess the DNA damage. The results were statistically compared using one-way Analysis of Variance (ANOVA).
Results
In the present study, the exposure of chick embryo brains to 2G and 3G cell phone radiation caused increased mean comet length (p<0.001), mean tail length (p<0.001), mean percentage of DNA in the tail (p<0.001) and mean tail moment (p<0.01) suggestive of increased DNA damage.
Conclusion
The present study concludes that the RFR exposure caused significant increase in DNA damage in developing brain of chick embryos with changes more pronounced in 3G exposure group.
Developing brain, Double strand breaks, Deoxyribonucleic acid, Radiofrequency radiation
Introduction
The 21st century has seen tremendous development in various fields of science and technology. With new inventions and devices that produce man-made electromagnetic fields, we have interfered too much with the natural environment causing unintended and undesirable negative impacts on the environment and living beings [1]. These inventions include cellular phones/mobile phones, wireless Local Area Network (wireless LANs), Bluetooth, Digital Enhanced Cordless Telecommunication (DECT), Ultra-Wide Band technology (UWB technology) and Wireless Power Transmission (WPT) from Solar Power Satellites (SPS). Among all these devices that emit man-made electromagnetic fields, the cellular mobile phone industry has undergone tremendous growth and development since its inception in Europe in early 1980’s. According to latest Groupe Speciale Mobile Association (GSMA) intelligence report, there are 5.16 billion cell phone users in the world with the number of smartphone users are increasing at a rate of 8% annually [2].
The cellular phone services require radiofrequency fields or high frequency electromagnetic fields which are a part of electromagnetic spectrum for transmitting and receiving signals. These fields cause the free radicals to stay longer within the cells and also alter the integrity of plasma membrane [3].
DNA, which is the highly stable macromolecules of the cell, is continually damaged by various endogenous factors (free radicals) and exogenous factors (UV rays, ionising and non-ionising radiation, chemicals and so on). The damaged DNA is usually repaired by DNA repair enzymes [4]. Any imbalance in DNA damage and its repair mechanisms or mistakes during repair may result in accumulation of damaged DNA resulting in cell death [5], ageing of the cell [6] or cancer [7]. The most common types of DNA damage are DNA strand breaks- Single Strand Breaks (SSB), Double Strand Breaks (DSB) and DNA cross links [4]. Most SSB’s are rapidly repaired with the intact strand serving as a template to direct rejoining process. On the other hand, DSB’s are more lethal and they are believed to be irreparable [8].
Various reports are available on the deleterious effect of RFR on DNA molecules on both animal models and human beings. The exposure of different animal models to RFR resulted in increased DNA strand breaks and rearrangement of DNA segments in various tissues like testis [9], brain [10], lung cells [11], embryonic stem cells [12] eyes [13] and liver [14].
Studies on various human cell cultures to RFR exposure have also shown an increased DNA damage-SSBs and DSBs. The RFR exposure ranging from 900-1800 MHz resulted in irreversible DNA damage in Human Lens Epithelial Cells (HLEC) [15,16], repairable DNA damage in HLECs [16,17], human fibroblasts [18], human lymphocytes [19], human hair root cells [20].
However, some studies have shown contradictory results in DNA damage on exposure to RFR fields in both animal models and human beings. No significant DNA damage was observed in murine C3H10T1/2 fibroblasts [21], Molt-4 cells [22], rat brain cells [23], spermatozoa of mouse caudal epididymis [24] and human lymphocyte cultures [25].
The various inconclusive controversial scientific reports and the rapid proliferation of cell phone industry going for a higher version of generation cell phones and their possible health impacts on the public has prompted us to undertake this research study. The present study was designed to evaluate the possible DNA damage to RFR exposure from 2G and 3G cell phone on developing and differentiating brain of the chick embryos using comet assay technique.
Materials and Methods
The experimental study has been carried out during the year August 2011-June 2015 and was designed according to the Ethical Guidelines for care and use of experimental animals. The protocol was approved by Institutional Animal Ethical Committee (IAEC). Fresh fertile hen eggs (Gallus domesticus) were procured from Rajiv Gandhi College of Veterinary and Animal Sciences, Puducherry, India. The eggs having approximately similar weight (65-70±5 gm) were selected for incubation in one particular batch. The eggs were incubated in six batches of 12 eggs each (total- 72 eggs) in a standard egg incubator at 37±0.5°c and 50-55% of humidity and ventilation. The eggs were rotated manually two times a day along the longitudinal and vertical axis and checked with a Candler for the viability of embryos. All live and healthy looking embryos showing normal curvature were included in the study. Dead embryos, embryos showing congenital anomalies and embryos without normal curvature were excluded from the study.
The first two batches of eggs (2×12=24 eggs) were grouped as sham exposed group (Group-C) and the eggs were incubated along with a popular brand cell phone with the Specific Absorption Rate (SAR) of 0.310 watts/kilogram hung from above with 5 cm distance separating the egg and kept in null status (switched off). Next two batches of fertilised eggs (2×12=24 eggs) were exposed to 2G cell phone radiation (Group-A) and last two batches of eggs (2×12=24 eggs) were exposed to 3G cell phone radiation (Group-B). They were incubated with the cell phone switched on and kept in silent mode. The head phone was plugged in to ensure the automatic activation of cell phone whenever it received a call and a radiofrequency meter (RF meter, Less EMF Inc, USA) was used to measure the intensity of radio frequency waves [Table/Fig-1].
A photograph showing the experimental set up. The mobile phone (red arrow) is hung with a distance of 5 cms separating it from the fertilised chicken eggs. A radiofrequency meter is kept inside the incubator to check the intensity of radiation (yellow arrow).

In the present study, same cell phone hand set and service provider were used for network connection for all the three groups. The first exposure was initiated at the 12th hour of incubation at 4.30 AM for 3 minutes duration period. Thereafter, on every half an hour lapse, the cell phone was rung for duration of three minutes each till 4.30 PM. Thus the embryos were exposed for 72 minutes duration over a 12 hour period (4:30 am-4:30 pm) followed by 12 hour of exposure-free period in a day. This was repeated regularly on subsequent days up to 12th day of incubation.
Three embryos per day were terminated from 9th day to 12th day. The brain were dissected out and minced in Hanks Balanced Salt Solution (HBSS w/ Phenol Red w/o ca and mg, Cat.No.55021C. SIGMA®). The cell suspension was used for the comet assay according to the protocol developed by Singh NP et al., [26].
The Single Cell Gel Electrophoresis (SCGE)/comet assay, developed by Singh NP et al., is a simple, reliable and sensitive technique used for quantification of low level DNA damage and repair in individual cells. The damaged DNA fragments with negative charge move outside the cell towards the anode leaving a trail resembling a comet’s tail and measurement of this tail gives the extent of DNA damage. The tail length of the comets is obtained by reducing the head diameter from total length of the comets [Table/Fig-2]. The low current used in this electrophoresis does not cause the movement of normal cell DNA. Thus, the degree of DNA damage can be quantified by this migration.
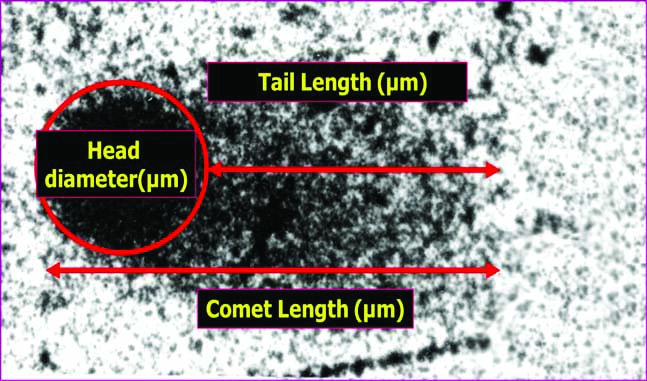
The slides were stained with silver nitrate with modifications in staining procedure [27]. Randomly selected 100 cells from the brain were then analysed using automated comet scoring software (Comet Score IV) to assess and quantify the levels of DNA damage in control group and both the experimental groups.
Statistical Analysis
The mean comet length, the mean tail length, mean percent of DNA in the tail and mean tail moment of all three groups were statistically compared using one-way ANOVA with Graph Pad Instat 3 and the significance was determined using a “Tukey’s post-hoc” with p<0.05 for statistical significance. All the data were expressed as Mean±SEM (Standard Error of Mean).
Results
i. Comet length
Both the 2G and 3G group embryos showed a extremely significant increase in comet length on comparing with control group embryos (p<0.001 and p<0.001, respectively). On comparing between 2G and 3G group embryos, the 3G group embryos showed statistically significant increase of comet length on 11th and 12th day (p<0.001 and p<0.001, respectively) [Table/Fig-3,4].
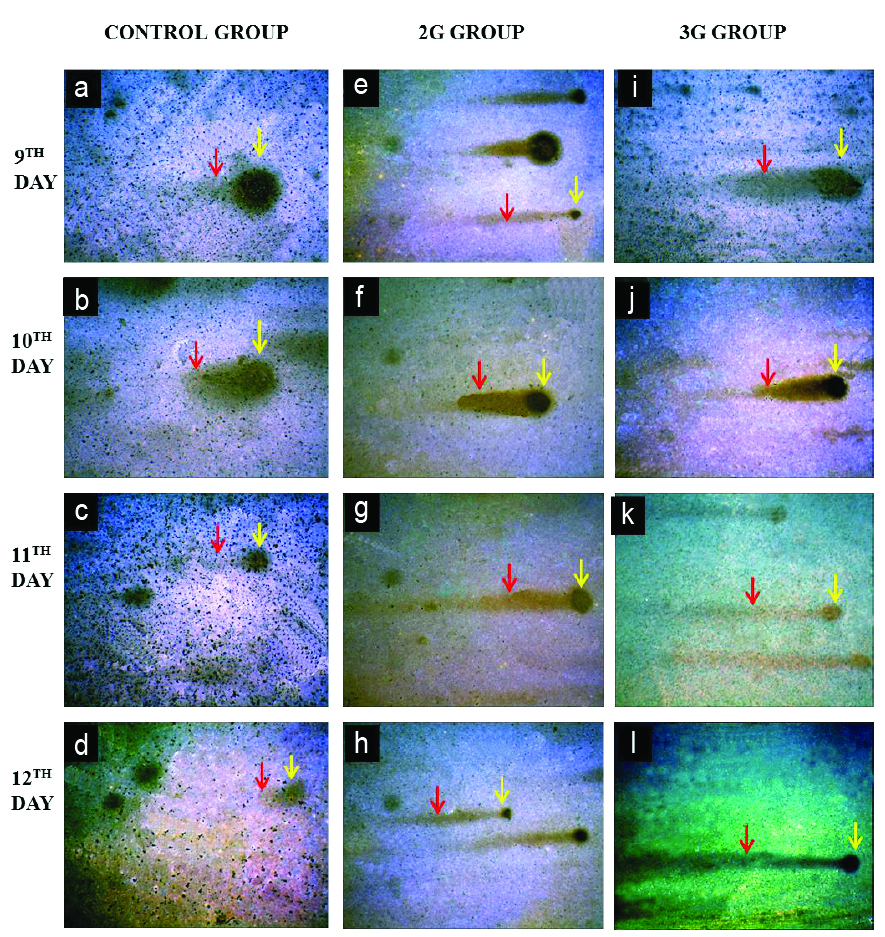
Photomicrograph showing comets in brain of control, 2G and 3G group embryos at 200X magnification (9th-12th day). Control embryo comets (a-d) showed minimal DNA damage with large head diameter (yellow arrow) and tail length is shorter (red arrow). Both 2G (e-h) and 3G group (i-l) embryos showed comets with severe DNA damage. Head diameter is decreased (yellow arrow) and tail length is increased indicating migration of damaged DNA (red arrow) (Silver nitrate staining).
Estimation of DNA damage in brain in all the 3 groups (9-12 days).
| Age in days | Mean comet length(μm) | Mean tail length(μm) | Mean % of DNA in tail | Mean tail moment(μm) |
|---|
| 9 (CON) | 5.5±0.13 | 4.15±0.08 | 24.88±1.1 | 119.1±6.9 |
| 9 (2G) | 7.4±0.22 | 5.48±0.2*** | 35.66±1.5*** | 169.9±9.1*** |
| 9 (3G) | 6.8±0.11 | 5.87±0.17*** | 35.31±1.5*** | 173.2±8.6*** |
| 10 (CON) | 5.5±0.13 | 4.17±0.1 | 25.72±1.1 | 123.8±6.8 |
| 10 (2G) | 6.5±0.20*** | 5.45±0.2*** | 33.73±1.4*** | 179.9±8.5*** |
| 10 (3G) | 6.7±0.14*** | 5.13±0.1*** | 41.03±1.6*** | 177.1±7.2*** |
| 11 (CON) | 4.9±0.09 | 3.12±0.08 | 34.9±2.19 | 149.7±7.3 |
| 11 (2G) | 6.5±0.20*** | 5.86±0.25*** | 48.56±2.8*** | 182.1±11.7 |
| 11 (3G) | 7.6±0.21*** | 6.2±0.2*** | 48.57±1.7*** | 250±16.07*** |
| 12 (CON) | 5.63±0.11 | 4.25±0.01 | 38.54±1.3 | 158.1±6.5 |
| 12 (2G) | 6.65±0.16*** | 4.89±0.14** | 55.39±1.3*** | 214.47±13.5** |
| 12 (3G) | 8.6±0.14*** | 6.5±0.13*** | 61.83±1.2*** | 267.6±9.5*** |
ANOVA followed by “Tukey’s post-hoc test was applied
Values are means±SEM taken from 3 samples per day for control, 2G and 3G group (n=36 chick embryos) (p-value <0.05* significant, <0.01 **highly significant and <0.001 ***extremely significant)
ii. Tail length
At 9th, 10th, 11th and 12th day of both the 2G (p<0.001, p<0.001, p<0.001 and p<0.01, respectively) and 3G group embryos (p<0.001, p<0.001, p<0.001 and p<0.001, respectively) showed significant increase in tail length of comets on comparing with control group embryos. On comparing between 2G and 3G group embryos, the 3G group embryos showed statistically significant increase of tail length on 12th day (p<0.001) [Table/Fig-3,4].
iii. Percentage of DNA in tail
The percent of DNA in the tail of comets in brain was also found to be significantly increased for both 2G and 3G group embryos in all the days (9th-12th days) when compared with the control group embryos (p<0.001 and p<0.001, respectively). On comparing between 2G and 3G group embryos, it was found that 3G group embryos showed increased percent of DNA in the tail than the 2G group embryos which was statistically significant on 10th and 12th days (p<0.001) [Table/Fig-3,4].
iv. Tail moment
Both the 2G and 3G group embryos showed an increase in the mean tail moment of comets when compared with the control group embryos. The increase was statistically significant for 2G group on 9th, 10th and 12th day (p<0.001, p<0.001, p<0.01, respectively) and for 3G group on all days (p<0.001). On comparing between 2G and 3G group embryos, 3G group embryos which showed a significant increase in tail moment on 11th day and 12th day (p<0.001 and p<0.01, respectively) [Table/Fig-3,4].
Discussion
Cells require a homeostatic environment for its survival and function. It maintains its homeostatic internal environment at the expenditure of energy (Na+ K+ ATPase pumps) by means of various control mechanisms [28]. Any alteration caused by various endogenous factors (free radicals) and exogenous factors (UV rays, ionising and nonionising radiation, chemicals and so on) to these control mechanisms could be fatal to the cells.
In the present study, all the parameters of the comets in brain were found to be increased in both the 2G and 3G group embryos that were significant statistically on comparing with control group embryos. Increased DNA damage in brain on RFR exposure was reported earlier by different authors. The exposure of mice to 2450 MHz microwaves resulted in increased DNA strand breaks and rearrangement of DNA segments in testis and brain [9]. The exposure of rat brain cells to a 2450 MHz RFR resulted in an increased SSB and DSB but the effects were blocked by antioxidants [10]. Their study suggested the role of free radicals in producing DNA strand breaks. Exposure of rat brain to 50 GHz microwaves resulted in increased DSBs [29]. Exposure of Wistar rats to RFR ranging from 915 MHz-2.45 GHz with SAR ranging from 0.6 W/kg-2.01 W/kg resulted in increased SSBs in brain [30].
However, these findings were contradicted by other researchers whose studies showed no significant effect of RFR producing DNA damage on brain. No significant change in DNA strand breaks, protein-DNA cross links, DNA–DNA cross links was observed in rat brain cells on exposure to 2450 MHz RFR [31]. The exposure of rat brain to 915 MHz to GSM mobile signal produced no significant DSB [23].
There are different hypothesis postulated by researchers regarding the interaction of electromagnetic radiations with DNA causing damages. Electromagnetic radiation consists of waves of both electric and magnetic energy that are detrimental to cellular safety. The energy associated with RFR (1.24.10-5 ev), cannot directly break the chemical bonds within the molecules to cause DNA damage. However, it increases the production of free radicals by means of Fenton reaction. In this reaction, hydrogen peroxides that are produced either by peroxisomes present in the cells or by dismutation of superoxide anion by Super Oxide Dismutase (SOD) [32] interacts with free iron to form highly reactive hydroxyl radicals (OH•)
Fe++ + H2O2 → Fe++++ HO + HO• (Fenton reaction)HO• ions are highly potent and gets added to DNA very rapidly in-vivo. They have very high electrophilicity and high reactivity to penetrate the shield of easily abstracted H atom in the sugar moieties and reach DNA bases. They thus attack DNA, yielding altered bases or SSB and DSB [28]. These free radicals are also known to produce damaging effects on macromolecules such as proteins and membrane lipids [33] causing structural alterations [34]. This probably could have caused structural alteration in DNA repair enzymes causing defective functioning of these enzymes leading to DNA damage [4]. Based on the present study and similar such scientific reports from different authors, we caution the public to use the cell phones judiciously till the scientific community comes out with a conclusive report on possible health effects of cell phone radiation. The already introduced 4G and much hurried roll out of 5G in certain countries ignoring the various scientific reports regarding the ill-effects of cell phone radiation from 2G and 3G cell phones, open a vast potential for future research.
Limitation(s)
The interaction of RFR with living tissues might vary from one species to another due to differences in volume and size, anatomical organisation of tissues, life span etc. Hence, direct extrapolation of results of present study on chick embryos to human population may be limited.
Conclusion(s)
The chronic exposure of developing chick embryos to RFR emitted from 2G and 3G cell phone resulted in DNA damage in the brain of both 2G and 3G group embryos with the damage more prominent in 3G group embryos. The DNA damage observed in the current study probably would have resulted from increased free radical production or due to structural alteration in DNA repair enzymes. Thus, by increasing the production of free radicals the external electromagnetic field produced from cell phones/mobile phones would have interacted with the internal biological processes of a cell, initiating a chain of reactions causing DNA damage.
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? No
For any images presented appropriate consent has been obtained from the subjects. No
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Oct 08, 2020
Manual Googling: Nov 24, 2020
iThenticate Software: Dec 15, 2020 (20%)
[1]. Orendacova J, Orendac M, Racekova E, Marsala J, Neuorobiological effects of microwave exposure: A review focused on morphological findings in experimental animalsArchives Italiennes deBiologie 2007 145:01-12. [Google Scholar]
[2]. We are social, Hootsuite, Kepios. “Digital 2020: April global stat shot”; retrieved from https://datareportal.com/reports/digital-2020-april-global-statshot [Google Scholar]
[3]. Ha BY, Stabilization and destabilization of cell membranes by multivalent ionsPhys Rev E Stat Nonlin Soft Matter Phys 2001 64(051902):01-05.10.1103/PhysRevE.64.05190211735963 [Google Scholar] [CrossRef] [PubMed]
[4]. Phillips JL, Singh NP, Lai H, Electromagnetic fields and DNA damagePathophysiology 2009 16(2-3):79-88.10.1016/j.pathophys.2008.11.00519264461 [Google Scholar] [CrossRef] [PubMed]
[5]. Schindowski K, Leutner S, Muller WE, Eckert A, Age related changes of apoptotic cell death in human lymphocytesNeurobiol Aging 2000 21:661-70.10.1016/S0197-4580(00)00171-8 [Google Scholar] [CrossRef]
[6]. Li H, Mitchell JR, Hasty P, DNA double-strand breaks: A potential causative factor for mammalian agingMech Aging Dev 2008 129:416-24.10.1016/j.mad.2008.02.00218346777 [Google Scholar] [CrossRef] [PubMed]
[7]. Ames BN, Endogenous DNA damage as related to cancer and agingMutat Res 1989 214:41-46.10.1016/0027-5107(89)90196-6 [Google Scholar] [CrossRef]
[8]. Anderson RE, Morgan B, Luis FF, Chapter 23. Radiation InjuryIn: Anderson’s Pathology (Ivan Damjanov and James Linder) 2009 vol.110th editionEl-sevier Inc:496-504. [Google Scholar]
[9]. Sarkar S, Ali J, Behari Effect of low power microwave on the mouse genome: A direct DNA analysisMutat Res 1994 320:141-47.10.1016/0165-1218(94)90066-3 [Google Scholar] [CrossRef]
[10]. Lai H, Singh NP, Effects of microwaves and a temporally incoherent magnetic field on single and double DNA strand breaks in rat brain cellsElectromag Biol Med 2005 24:23-29.10.1081/JBC-200055046 [Google Scholar] [CrossRef]
[11]. Zhang DY, Xu ZP, Chiang H, Lu DQ, Zeng QL, Effects of GSM 1800 MHz radiofrequency electromagnetic fields on DNA damage in Chinese hamster lung cellsZhonghua Yu Fang Yi Xue Za Zhi 2006 40:149-52. [Google Scholar]
[12]. Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cellsFASEB J 2005 19:1686-88.10.1096/fj.04-3549fje16116041 [Google Scholar] [CrossRef] [PubMed]
[13]. Mary HD, Rijied TS, Anbalagan J, Rajesh B, Effect of ultra high frequency radiation from 2G and 3G cell phone on histology of chick embryo retina- A comparative studyInternational Journal of Science and Research 2015 4(2):1639-52. [Google Scholar]
[14]. Mary HD, Rijied TS, Anbalagan J, Rajesh B, Effect of radiofrequency radiation emitted from 2G and 3G cell phones on developing liver of chick embryo- A comparative studyJ Clin Diagn Res 2017 11(7):AC05-09. [Google Scholar]
[15]. Lixia S, Yao K, Kaijun W, Effects of 1.8 GHz radiofrequency field on DNA damage and expression of heat shock protein 70 in human lens epithelial cellsMutat Res 2006 602:135-42.10.1016/j.mrfmmm.2006.08.01017011595 [Google Scholar] [CrossRef] [PubMed]
[16]. Sun LX, Yao K, He JL, Lu DQ, Wang KJ, Li HW, Effect of acute exposure to microwave from mobile phone on DNA damage and repair of cultured human lens epithelial cells in vitroZhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2006 24:465-67. [Google Scholar]
[17]. Yao K, Wu W, Wang K, Electromagnetic noise inhibits radiofrequency radiation induced DNA damage and reactive oxygen species increase in human lens epithelial cellsMol Vis 2008 14:964-69. [Google Scholar]
[18]. Diem E, Schwarz C, Adlkofer F, Jahn O, Rudiger H, Non-thermal DNA breakage by mobile-phone radiation (1800-MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitroMutat Res 2005 583:178-83.10.1016/j.mrgentox.2005.03.00615869902 [Google Scholar] [CrossRef] [PubMed]
[19]. Markova E, Hillert L, Malmgren L, Persson BR, Belyaev IY, Microwaves from GSM mobile telephones affect 53BP1 and gamma-H2AX foci in human lymphocytes from hypersensitive and healthy personsEnviron Health Perspect 2005 113:1172-77.10.1289/ehp.756116140623 [Google Scholar] [CrossRef] [PubMed]
[20]. Çam ST, Seyhan N, Single-strand DNA breaks in human hair root cells exposed to mobile phone radiationInt J of Radiat Biol 2012 88(5):420-24.10.3109/09553002.2012.66600522348707 [Google Scholar] [CrossRef] [PubMed]
[21]. Li L, Bisht KS, Lagroye I, Zhang P, Straube WL, Moros EG, Measurement of DNA damage in mammalian cells exposed in vitro to radiofrequency fields at sars of 3-5 W/kgRadiat Res 2001 156:328-32.10.1667/0033-7587(2001)156[0328:MODDIM]2.0.CO;2 [Google Scholar] [CrossRef]
[22]. Hook GJ, Zhang P, Lagroye I, Li L, Higashikubo R, Moros EG, Measurement of DNA damage and apoptosis in molt-4 cells after in vitro exposure to radiofrequency radiationRadiat Res 2004 161:193-200.10.1667/RR312714731070 [Google Scholar] [CrossRef] [PubMed]
[23]. Belyaev IY, Koch CB, Terenius O, Exposure of rat brain to 915 MHz GSM microwaves induces changes in gene expression but not double stranded DNA breaks or effects on chromatin conformationBio Electromagnetics 2006 27:295-306.10.1002/bem.2021616511873 [Google Scholar] [CrossRef] [PubMed]
[24]. Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV, Impact of radio frequency electromagnetic radiation on DNA integrity in the male germlineInt J Androl 2005 28:171-79.10.1111/j.1365-2605.2005.00531.x15910543 [Google Scholar] [CrossRef] [PubMed]
[25]. Zeni O, Romano M, Perrotta A, Lioi MB, Barbieri R, d’Ambrosio G, Evaluation of genotoxic effects in human peripheral blood leukocytes following an acute in vitro exposure to 900 MHz radiofrequency fieldsBioelectromagnetics 2005 26(4):258-65.10.1002/bem.2007815832336 [Google Scholar] [CrossRef] [PubMed]
[26]. Singh NP, McCoy M, Tice RR, Schneider EL, A simple technique for quantitation of low levels of DNA damage in individual cellsExperimental Cell Research 1988 175(1):184-91.10.1016/0014-4827(88)90265-0 [Google Scholar] [CrossRef]
[27]. Rajesh B, Detection of level of DNA damage in petrol pump attendants of Puducherry union territory through Comet AssayDSTE-Project Report 93, Department of Science, Technology & Environment, Pondicherry, India 2014 http://dste.puducherry.gov.in/DSTE-Final-report-Dr.Rajesh.pdf [Google Scholar]
[28]. Anjana VY, David GK, Janardan KR, Chapter 17. Cell Injury and cellular adaptationsIn: Anderson’s Pathology (Ivan Damjanov and James Linder) 2009 110th editionEl-sevier Inc:361-66. [Google Scholar]
[29]. Kesari KK, Behari J, Fifty gigahertz microwave exposure effect of radiations on rat brainAppl Biochem. Biotechnol 2009 158:126-39.10.1007/s12010-008-8469-819089649 [Google Scholar] [CrossRef] [PubMed]
[30]. Trosic I, Pavicic I, Milkovic KS, Mladinic M, Zelijeric D, Effect of electromagnetic radio frequency radiation on rats brain, liver and kidney cells measured by comet assayColl Antropol 2011 35(4):1259-64. [Google Scholar]
[31]. Lagroye I, Anane RR, Wettring BA, Moros EG, Straube WL, Laregina M, Measurement of DNA damage after acute exposure to pulsed-wave 2450 MHz microwaves in rat brain cells by two alkaline comet assay methodsInt J Radiat Biol 2004 80:11-20.10.1080/09553000310001642911 [Google Scholar] [CrossRef]
[32]. Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J, Role of oxygen radicals in DNA damage and cancer incidenceMol Cell Biochem 2004 266:37-56.10.1023/B:MCBI.0000049134.69131.8915646026 [Google Scholar] [CrossRef] [PubMed]
[33]. Lai H, Singh NP, Magnetic-field-induced DNA strand breaks in brain cells of the rat, EnvironHealth Perspect 2004 112:687-94.10.1289/ehp.635515121512 [Google Scholar] [CrossRef] [PubMed]
[34]. George DF, Bilek MM, McKenzie DR, Non-thermal effects in the microwave induced unfolding of proteins observed by chaperone bindingBioelectromagnetics 2008 29:324-30.10.1002/bem.2038218240290 [Google Scholar] [CrossRef] [PubMed]