The present study was undertaken to analyse the epidemiological profile, hormone receptor and HER2/neu receptor response after platinum based compound added to neoadjuvant chemotherapy (NACT) in LABC of TNBC cases in comparison to patients of TNBC who did not receive any chemotherapy. While molecular characteristic is not a standard of care, but platinum based therapy often offer pathological partial response to these TNBC patients.
Materials and Methods
An institutional based retrospective observational study was conducted in the Department of Pathology at Kolkata which is a Tertiary Care Centre of West Bengal from hospital database registered during 3 years (January 2017 to December 2019). Total 390 breast cancer patients were registered in this period, among which 104 TNBC patients were selected for study. The present study was done only with TNBC patients which is considered to be associated with aggressiveness and poor prognosis.
All the selected TNBC (104) patients showing LABC had trucut biopsy with IHC check for ER, PR, HER2/neu receptors in all the cases. Thereafter, the patients underwent modified radical mastectomy. They were subdivided into two groups- as study group (NACT-TNBC) comprising of patients who received platinum based chemotherapy before radical mastectomy with pathological partial response after chemotherapy and those in the control group (non NACT-TNBC) that included patients who did not received any chemotherapy but had modified radical mastectomy. IHC was again carried out on formalin fixed paraffin block embedded sections taken from all 104 mastectomy specimen.
Inclusion criteria: A total of 104 patients of LABC with TNBC were included in the study, among which 40 cases were considered as NACT-TNBC group who had received 4-6 cycles of NACT comprising of 5- Fluorouracil, doxorubicin and cyclophosphamide along with carboplatin followed by surgery (MRM) and had residual tumour with Miller-Payne grade ≤3. The other non NACT-TNBC control group comprised of 64 LABC patients who did not received any NACT and mastectomy was performed to prevent recurrence. All patients were negative for HRs and HER2/neu in both NACT and non NACT groups in their trucut biopsy report before mastectomy. The breast specimens from both the groups were grossed as per protocols and section from paraffin blocks were stained by Haematoxylene and Eosin (H&E) for histo-pathological grading. Nottingham’s histologic scoring was done. The response to NACT was categorised according to the Miller-Payne classification [5]. Reporting was done by Pathology experts of the same institute.
Exclusion criteria: The cases showing complete pathological response in residual tumour after NACT and of breast cancers with distant metastasis were excluded from present study.
Masterchart was prepared comprising patient’s age, menopausal status, family history, therapy history, histo-morphological features, and hormone receptor and HER2/neu status after platinum added chemotherapy. ER/PR were considered positive, if >1% tumour cell nuclei were immunoreactive and negative, if it was otherwise. For HER2/neu study, kits approved by Food and Drug Administration (FDA) were used and scoring was done as per Hercep Test guidelines [6]. HER2/neu score of 3+ was taken as positive by immunohistochemistry method. For equivocal results (2+), ISH (in-situ hybridisation) tests were advised. Due to lack of patients’ compliance, survival analysis could not be carried out. Breast Cancer (BRCA) gene status of all the patients was not done as of limited resource.
Statistical Analysis
Data was entered in MS excel. For descriptive purposes, percentages were calculated. Comparison of both groups was done by Pearson’s Chi-squared or Fisher’s-exact test. Intergroup p-value was calculated by Fisher’s-exact test for 2×2 contingency table. Parameters were studied on categorical scale between two or more groups, using Statistical Package for the Social Sciences (SPSS)-18. Significance level was considered at p-value <0.05.
Results
Out of 390 breast cancer patients, registered during the period of 3 years, 104 (104/390=26.6%) patients were found to be TNBC. Patients were selected based on inclusion criteria from January 2017 to December 2019 from the registered records. The mean age of TNBC patients belonged to study group was 43 years with range of 17 to 78 years. The mean age for control group was 51 years with age range of 33 to 76 years. Considering 46 years as current age for menopause [7], majority of patients (25 or 62.50%) were pre-menopausal in the study group while in the control group, majority breast cancer patients were belonged to postmenopausal (35 or 54.69%). The difference was statistically not significant. The Chi-square test result was 2.91, p=0.08 (non significant) [Table/Fig-1].
Showing comparison of menopausal status.
| Age | NACT-TNBC | Non NACT-TNBC |
|---|
| ≤46 years | 25 (62.50%) | 29 (45.31%) |
| >46 years | 15 (37.50%) | 35 (54.69%) |
| Total | 40 | 64 |
NACT-TNB: Neoadjuvant chemotherapy-Triple negative breast cancer
Comparison of the two groups were made based on the risk factors using age of these patients, family history of cancer, age of first child birth and breast feeding history [Table/Fig-2]. It was observed that mean age of the patients, age of first child birth and breast feeding history were higher in the non NACT-TNBC group.
Comparison of other risk factors between study and control group of TNBC patients.
| Risk factors | NACT-TNBC (40) | Non NACT-TNBC (64) |
|---|
| Mean age (Years) | 43 | 51 |
| Family history | 05 | 03 |
| Breast feeding | 31 (77.50%) | 55 (85.9%) |
| Mean age of first child birth | 21 years | 24 years |
NACT-TNBC: Neoadjuvant chemotherapy-Triple negative breast cancer
Most of the patients had histological grade 2 type breast carcinoma (24 NACT-TNBC=60%) and constituting almost 59% in both groups irrespective of their histological type. The study found 10 (25%) cases of grade 3 tumour in study group. Post-NACT patients showed conversion of histological grade 2 to grade 1 in 5 (12.5%) cases and from grade 3 to grade 2 in 16 (40%) cases [Table/Fig-3]. This study showed that Nottingham grade LABC was statistically associated with TNBC-which suggested aggressive behaviour of TNBC (The Chi-square statistic was 4.45, the p-value was 0.035: Significant at p<0.05). [Table/Fig-4]. A standard uniform protocol of fixation, antigen recovery, and same antibody clone with same dilution, uniform time and temperature incubation for IHC was followed. The IHC was repeated in the discordant cases and using positive controls of ER, PR and HER2/neu [Table/Fig-5]. An alteration of 17 (42.5%) in ER, 4 (10%) in PR and 9 (22.5%) in HER2/neu receptor was observed among NACT-TNBC group. The discordance in non NACT-TNBC group was 3 (5%), 2 (3%), and 5 (8%), respectively. Criteria of Allred score ≥3 for HR positivity and 3+ as per four graded system for HER2/neu positivity was applied. The effect of NACT was found to be statistically significant with respect to change in HER2/neu (p-value=0.033, p<0.05) and ER status (p<0.05) while PR was found to be statistically insignificant [Table/Fig-6].
Comparison of Nottingham histological grade and Conversion of Grade after NACT.
| Nottingham grade | NACT-TNBC (40) | Non NACT-TNBC (64) | Others Non TNBC group (286) |
|---|
| Grade 1 | 06 (15%) | 01 (02%) | 31 (11%) |
| Grade 2 | 24 (60%) | 38 (59%) | 189 (66%) |
| Grade 3 | 10 (25%) | 25 (39%) | 66 (23%) |
| Conversion of Grade after NACT |
| Grade 2 to 1 | 05 (12.5%) |
| Grade 2 to 3 | 01 (2.5%) |
| Grade 3 to 1 | 01 (2.5%) |
| Grade 3 to 2 | 16 (40%) |
NACT: Neoadjuvant chemotherapy; TNBC: triple negative breast cancer
Association of Nottingham grade Locally Advanced Breast Cancer with TNBC-which suggested aggressive behaviour of TNBC.
| Nottingham grade | TNBC | Others | Total | Chi-square test | p-value |
|---|
| 1 and 2 | 69 | 220 | 289 | 4.45 | 0.035: Significant |
| 3 | 35 | 66 | 101 |
| Total | 104 | 286 | 390 |
TNBC: Triple negative breast cancer
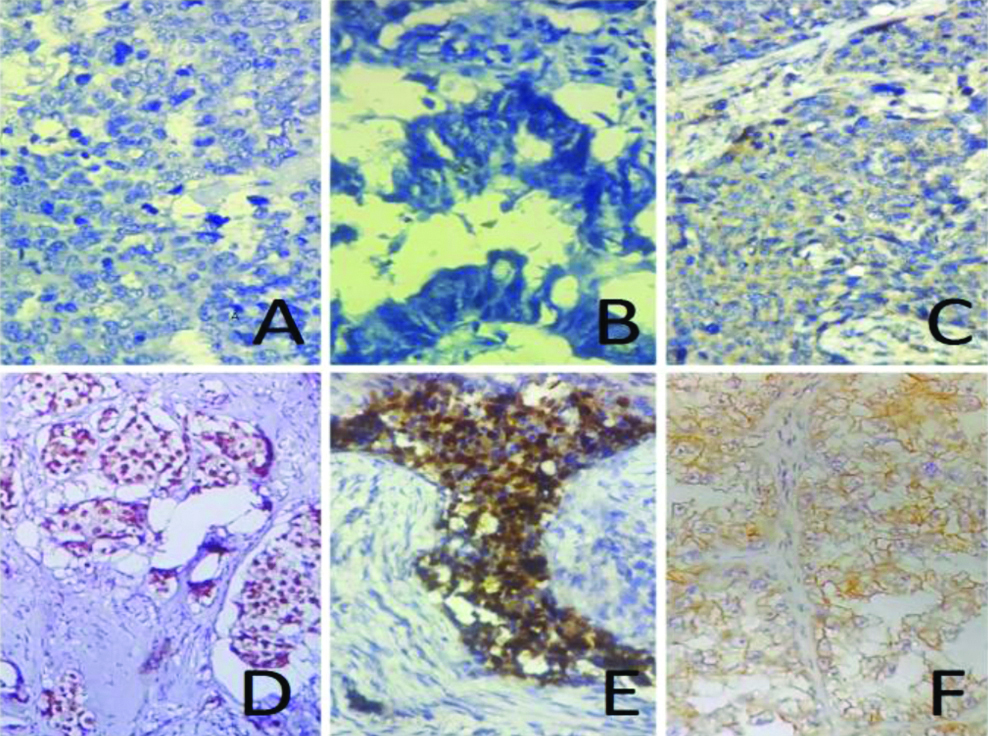
Microphotographs of Triple Negative Breast Cancer (TNBC): A) ER Negative (400X Magnification); B) PR Negative (400X); C) HER2/neu Negative (400X) Haematoxylene was used as counter stain. Representing both the test group (NACT-TNBC) and control group (non NACT-TNBC); D) Positive Control of ER (400X); E) Positive control of PR (400X); and F) Positive control of HER2/neu (400X). The stain used for positive controls is Horseradish Peroxidase (HRP) in secondary antibody.
The effect of NACT was found to be statistically significant with respect to change in HER2/neu and ER status while PR is found to be statistically insignificant.
| Receptor status | NACT-TNBC | Non NACT-TNBC | Total | Chi-square test | p-value |
|---|
| ER change (Negative to positive) | 17 (42.5%) | 3 (5%) | 20 | 22.66 | <0.00001 Significant |
| No change in ER | 23 | 61 | 84 |
| Total | 40 | 64 | 104 |
| PR change (Negative to positive) | 4 (10%) | 2 (3%) | 6 | 2.14. | 0.1Non significant |
| No change in PR | 36 | 62 | 98 |
| Total | 40 | 64 | 104 |
| HER2/neu change (Negative to positive) | 9 (22.5%) | 5 (8%) | 14 | 4.56 | 0.033 Significant |
| No change in HER2/neu | 31 | 59 | 90 |
| Total | 40 | 64 | 104 |
| Total discordance (In ER, PR or HER2/neu change) | 30 | 10 | 40 | 36.66 | <0.00001 Significant. |
| No change in any receptor | 10 (25%) | 54 (84%) | 64 |
| Total | 40 | 64 | 104 |
NACT: Neoadjuvant chemotherapy; TNBC: Triple negative breast cancer; ER: Oestrogen receptor; PR: Progesterone receptor
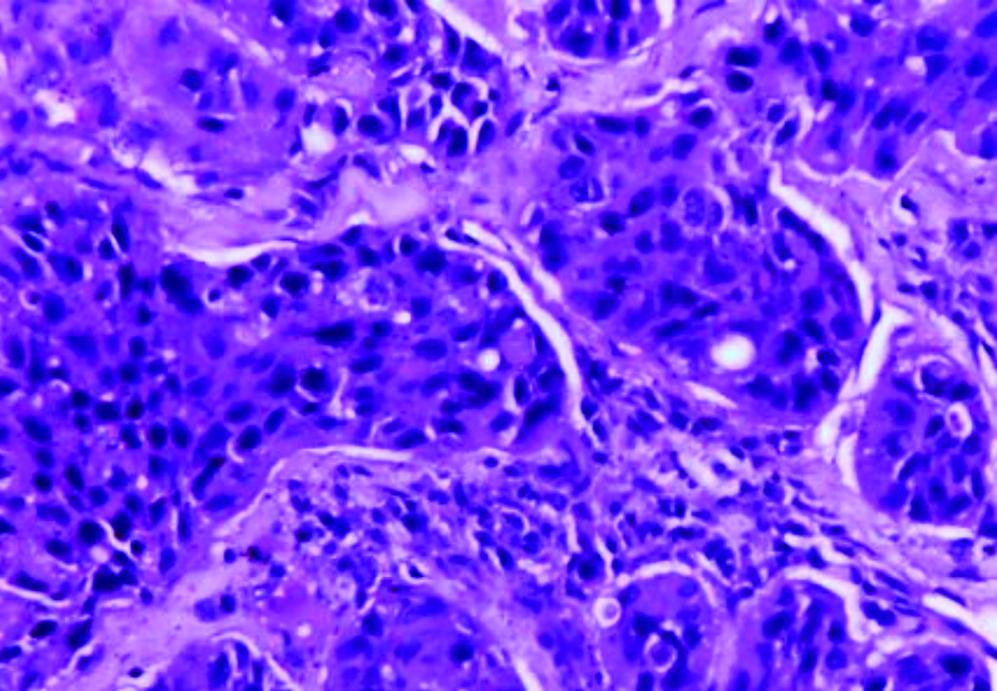
All the patients belonging to both groups had LABC having T stage ≥T3 and/or N-stage ≥N2 without any evidence of distant metastasis. Among both groups, the common variant was Invasive Ductal Carcinoma, Not Otherwise Specified (IDC, NOS) [Table/Fig-7]. Cases of IDC-NOS, metaplastic carcinoma, medullary carcinoma, invasive lobular carcinoma, sebaceous carcinoma, encapsulated papillary carcinoma in the NACT-TNBC group constituted 32 (80%), 2 (5%), 3 (7.5%), 2 (5%), 0, 1 (2.5%) and in the non NACT-TNBC included 58 (91%), 1 (1.5%), 1 (1.5%), 2 (3%), 1 (1.5%) and 1 (1.5%), respectively. IDC-NOS comprised of tumours sheets, nests, cords or individual cells. Tubular formations were prominent in well differentiated tumours but absent in poorly differentiated tumours [Table/Fig-7]. Stroma was usually desmoplastic. Often DCIS and perineural invasion (28%) were present. Mitotic figures were often prominent particularly in high grade tumours.
Invasive Ductal carcinoma, not otherwise specified (IDC, NOS)- high grade which on IHC showed Triple negative breast carcinoma (400X).
Discussion
The present study is a retrospective evaluation of the presence of TNBC in an Eastern Indian population and its comparison with risk factors, histological features, expression of HER2/neu and other hormonal status. Among the subgroups of breast cancer, high aggressive behaviour and poor clinical outcome of TNBC raises the fear because specific chemotherapy regimen for the successful treatment of TNBC has not been recommended yet, Platinum-based regimens have been suggested to possibly be more active in TNBC [8]. Other parameters like the Tumour-Infiltrating lymphocytes (TILs), reactive atypia, retraction artefact status, small cell like features, level of tumour necrosis and clear cytoplasm were found unnecessary to depict here, as post-NACT morphological changes was not the objective of the study. This study appears to be first study in Eastern India where changes of hormonal and HER2/neu receptor changes were observed in TNBC patients following platinum added chemotherapy.
Among the previous studies, the TNBC occurrence was accounted for almost 26% to 27.9% of all breast cancer [9,10]. The young black women [11,12] was found to be more affected along with poor prognosis. The current study also showed the prevalence of TNBC of 26.6% which suggests higher value of affliction in Indian populations [13-15]. In India, the incidence of TNBC varies from 11.2% to 29.8% [16-18]. The mean age of patients of present study group was 43 years, which in comparison to the western data is much younger as noted by earlier works [9,18]. Younger mean age in Indian people suggests the general trend of breast cancers occurring a decade earlier in contrast to the western population [16]. In this study, premenopausal cases were more in number (62.50%) than the postmenopausal patients in the test group, whereas postmenopausal patients were more (54.69%) in control group. The present study result was supported by two previous Indian studies by Lakshmaiah KC et al., and Akhtar M et al., [9,15]. The reason behind the TNBC patients not receiving chemotherapy may be due to their late age, their medical unfitness to chemotherapy, uncontrolled diabetes, dropout cases as patient’s or patient party’s incompliance to allopathic chemotherapy. TNBC is still thought as waste basket group, not even considered suitable for chemotherapy which may be one of the reasons, why present study got large control group (64) who did not receive any chemotherapy. Previous study by Das R et al., on the predictive and prognostic behaviour of breast cancer by NACT on LABC cases not exclusive of TNBC, showed significant alteration of hormone receptors and HER2/neu expression in the biopsy specimens of the patients after receiving NACT [5].
The majority of TNBC tumours of both study and control group were of higher grades. These features are consistent with other available studies [15,19-21]. Statistical analysis was observed with Chi-square test. Significant association was found between higher tumour grade and unfavourable receptor expression. The p-value is 0.035 (significant at p<0.05) [Table/Fig-4]. Nottingham grade LABC was statistically associated with TNBC-suggesting aggressive behaviour of TNBC.
Before the introduction of NACT, prognosis of the LABC was very poor. With the widespread use of NACT in cases of TNBC, the effect of this therapy on ER, PR, and HER2/neu receptor status has been questioned. This study was undertaken to determine the alteration in HR and HER2/neu receptor status following NACT in study group and also to compare the findings with patients operated without NACT (control group). This discordance in non NACT group may probably be caused by intra-tumoural heterogeneity or due to intra- and inter-observer variability.
Among previous two Indian studies, one study showed that in 8% of cases, ER changed from negative to positive status and in 15% cases, PR changed from negative to positive, out of a total of 73 cases [22,23]. The present study also observed considerable HER2/neu status changes (22.5% negative to positive) after NACT in contrary to the findings in previous study, ‘HER2/neu expression rarely changes after chemotherapy [24]. The discordance in HR and HER2/neu status among the study group who received chemotherapy could be due to direct effect of NACT targeting heterogeneous chemosensitive tumour cells, leaving behind insensitive tumour cells with different biology in the residual disease [24]. PR receptor status was relatively unaffected by NACT and suggests poorer prognosis possibly due to tumour heterogeneity. The present study finding corroborated to the clinical outcome of observations made by Biswas T et al., that suggested pathological Complete Response (pCR) following NACT results in improved survival among patients with TNBC and is independent of diagnostic stage [25].
Limitation(s)
The prime limitation of this study was small sample size of TNBC for platinum based chemotherapy. The other limitations are exclusion of four cases of TNBC with distant metastasis. The large scale prospective trial of platinum based chemotherapy among all types TNBC patients, along with gene expression profile and expression of basal cell markers are required to know the exact behaviour of these notorious tumours and to reveal the targeted regimen.
Conclusion(s)
TNBCs are highly aggressive subtype with high grade and more common in young aged patients. Although current guidelines do not recommend repeat performance of hormonal and HER2/neu receptors status following NACT, the present study observed significant alteration in hormonal and HER2/neu receptor status in TNBC patients receiving platinum added chemotherapy. It is known to all that TNBC has limited treatment options and very poor prognosis following progression after standard anthracycline or taxane regimens. TNBCs are more common in our country than the western literature. This study found statistical significance and justifies re-evaluation of these HR and HER2/neu markers in residual tumour after chemotherapy. Larger studies to analyse the impact of these alteration and its significance in patient survival need to be considered in patients’ treatment benefit.
NACT-TNB: Neoadjuvant chemotherapy-Triple negative breast cancer
NACT-TNBC: Neoadjuvant chemotherapy-Triple negative breast cancer
NACT: Neoadjuvant chemotherapy; TNBC: triple negative breast cancer
TNBC: Triple negative breast cancer
NACT: Neoadjuvant chemotherapy; TNBC: Triple negative breast cancer; ER: Oestrogen receptor; PR: Progesterone receptor