Malaria remains a key universal health problem affecting about 228 million people resulting in 4.05 lacs deaths according to the World Health Organisation (WHO) report 2019 [1]. The South-East Asia Region (SEAR) accounted for 3.4% of global cases of malaria following African Region (93%) in 2018. In 2018, most of deaths occurred in the African Region (94%) followed by India (3%). According to the World Malaria Report 2019, incidence of malaria in India accounted for 68% of cases and 65% of malaria deaths in the SEAR [2]. Plasmodium falciparum causes various complications and is the cause of almost all deaths due to malaria. AKI occurs most commonly in Plasmodium falciparum infection. Ten years back, cerebral malaria was the principle manifestation of severe falciparum malaria, whereas now multiple complications are more common with the constellation of cerebral malaria, jaundice and renal failure [3,4]. Next to cerebral malaria and anaemia, AKI is the third most common complication of falciparum malaria. Several factors have been implicated in the pathogenesis of AKI in malaria. Falciparum malaria has been connected with an increase in oxidative stress, in relation with disease severity [5,6]. NAC, a thiol containing antioxidant has been shown to ameliorate ischemic renal failure in animals and has been used in humans to prevent a reduction in renal function in patients with acetaminophen induced liver failure [7]. Based on these observations, several clinical studies [8-11] have evaluated the efficacy of NAC in the prevention of radio contrast nephropathy. NAC inhibits TNF and impedes cytoadherence implicated in the pathogenesis of malaria complications [12,13].
Odisha is the most malaria endemic state in India, accounting for a quarter of all cases; 47% of all P.falciparum cases reported in India occur here [26]. In the year 2014, 13.64% (73) of total death due to malaria across the country was from Odisha [27]. Growing insight into mechanism progressing to AKI and the significance of facilitating renal improvement has encouraged investigators to assess the role of newer therapeutic agents in the prevention of AKI. So, the study was conducted to study the effect of NAC on improvement and deterioration of falciparum malarial AKI.
Materials and Methods
The present prospective observational study was undertaken in patients of falciparum malaria with AKI who were admitted to the Department of General Medicine ward, VSSIMSAR, Burla, Odisha from November 2014 to October 2016. This study was conducted according to the principles expressed in the declaration of Helsinki and approved by the ethics committee (Regn. No. ECR/861/Inst/OR/2016) (Communication on VIREC Decision- No. 2014/P-I-RP/14M-O-MED-018/058). Written informed consent was obtained in the prescribed format from parents and guardians of all patients included in this study. The total number of cases included at the beginning was 176, and those remaining after inclusion and exclusion criteria were applied in this study were 100.
Inclusion criteria: Patients aged >14 years of age, falciparum malaria cases with AKI who were admitted and patients with falciparum malaria and AKI who refused haemodialysis were included in the study.
Exclusion criteria: Patients with history of chronic kidney disease, h/o end stage renal disease, history of any other prior obvious renal abnormality such as polycystic kidney disease, pregnancy and lactation, patients who refused to consent for the study, patients who required haemodialysis, patients who died during initial five days of the study, and patients who Left Against Medical Advice (LAMA) within five days of the study were excluded from the study.
Patients with falciparum malaria diagnosed either by Quantum Buffy Coat (QBC) or Immunochromatographic Antigen-Detection Test (ICT) or slide (thick and thin smear) test with AKI who were treated in the Department of General Medicine VSSIMSAR, Burla were taken in this study and were divided into two groups based on NAC treatment [28].
Patients, selected alternatively, who were treated with NAC were considered as NAC group and those who were not given were considered as NNAC group. Both the groups were given standard treatment including intravenous artesunate as per WHO guidelines [29]. In treatment group (NAC group), NAC was given 600 mg twice daily per orally or through Ryle’s tube for five days and in control group (NNAC group), NAC were not given and both the groups were compared. Age and gender details as well as weight of the patients were recorded.
In the groups, serum creatinine level and urine output were compared on day 1, day 3 and on day 5 of the study. The percentage increment and decrement in serum creatinine level was calculated on day 5 with respect to day 1.
Those who had increment in serum creatinine on day 5 with respect to day 1 were considered as deterioration in AKI and those who had decrement in serum creatinine on day 5 with respect to day 1 were considered as improvement in AKI.
The study estimated the serum cystatin-C level in all cases on the first day of admission. Serum cystatin-C more than 1.0 mg/dL was considered abnormal [30]. Those who died or required haemodialysis or refused treatment within five days were excluded from the study. Age and weight were recorded for all the remaining participants.
Both NAC and NNAC group were further subjected to the following investigations: total bilirubin, haemoglobin, serum creatinine, urine output. The mean duration of fever at the time of presentation was also recorded.
Acute Kidney Injury was defined as per the new criteria, Kidney Disease Improving Global Outcomes (KDIGO) criteria mentioned below taking into consideration of both serum creatinine and urine output [31]. AKI is defined as any of the following: Increase in serum creatinine by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 hours; or increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior seven days; or urine volume <0.5 mL/kg/h for 6 hours [31].
KDIGO Staging of AKI-
Stage-1:
Serum creatinine 1.5-1.9 times baseline or
≥0.3 mg/dL increase with/or urine output <0.5 mL/kg/h for 6-12 hours
Stage-2:
Serum creatinine 2-2.9 times baseline with or
Urine output <0.5 mL/kg/h for >12 hours
Stage-3:
Serum creatinine 3 times baseline or
Increase in Serum creatinine to ≥4 mg/dL with or
Initiation of renal replacement therapy with urine output <0.3 mL/kg/h for ≥24 hour or
Anuria for ≥12 hour
As some of the patients referred from peripheral hospital to our hospital, had already done their serum creatinine including other blood tests; so when the basal serum creatinine was known, it was easy to define AKI and on which stage, it would be on day 1, otherwise when the basal creatinine was not known and serum creatinine could not be done on day 1, AKI would be defined from urine output according to KDIGO criteria.
Statistical Analysis
The data was collected and entered in a predesigned Microsoft office excel format which was used for various calculation and statistical analysis. Graph pad instat version-3 for window was used for various statistical analyses. The numerical values were compared by Chi-square test. The comparison of mean values among the NAC and NNAC groups was performed by student’s t-test. The comparison of mean value of different parameters among more than two groups was done by one way analysis of variance (ANOVA). A p-value of <0.05 was considered as statistically significant.
Results
In this prospective hospital based observational study, 176 cases had falciparum malarial AKI during the stipulated study period. Of which 94 cases were given NAC, designated as NAC group, along with standard antimalarial treatment. Out of 94 cases who were given NAC, 29 (30.85%) cases needed haemodialysis within five days of this study and 12 patients became LAMA and 3 (3.19%) patients died within five days of the study. Based on exclusion criteria, all these patients were excluded from the study. The remaining 50 cases, those were treated with NAC for five days along with other standard treatment including intravenous artesunate were taken into the study as NAC group. Out of 82 cases who did not take NAC, designated as NNAC group, 26 (31.70%) cases needed haemodialysis, two became LAMA and 4 (4.8%) died within five days of the study. Then only 50 cases in NNAC group were treated with intravenous artesunate for five days along with other standard treatment without giving NAC. So, the study subjects included total 100 cases of falciparum malaria with AKI as per inclusion criteria mentioned above.
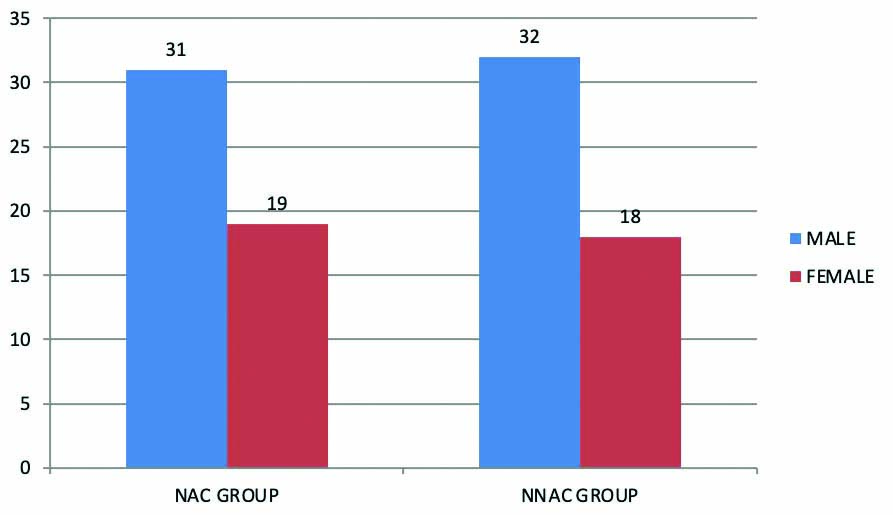
Among 50 cases in NAC group, 31 were males and 19 were females and in 50 cases of NNAC group 32 were males and 18 were females constituting 63 males (63%) and 37 females (37%) in total of 100 cases [Table/Fig-1].
Gender distribution in NAC and NNAC group.
NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine
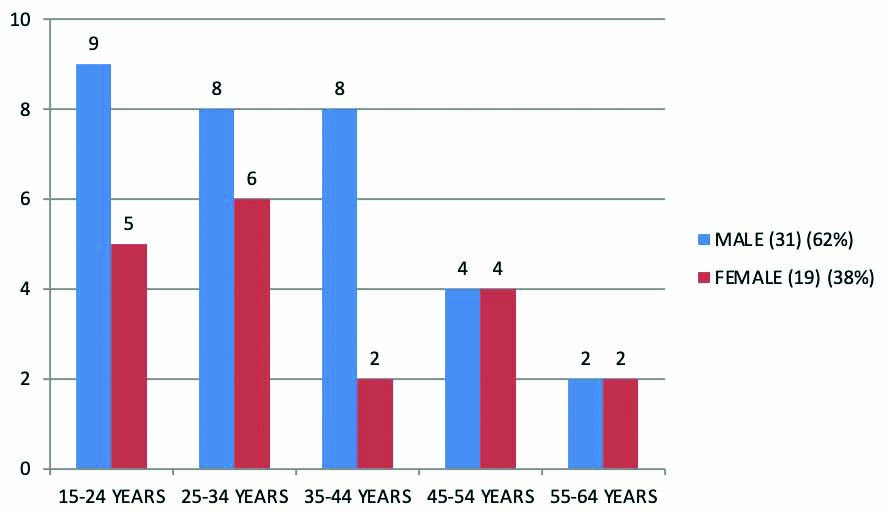
Among the 50 cases in NAC group, 14 cases (28%) were present in 15-24 years age group, 14 cases (28%) were in 25-34 years age group, 10 cases were in 35-44 years age group, eight cases were in 45-54 years age group and four cases were more than 54 years. Most of the cases were present in 15-34 years age group [Table/Fig-2].
Age distribution in NAC group.
NAC: N-Acetylcysteine
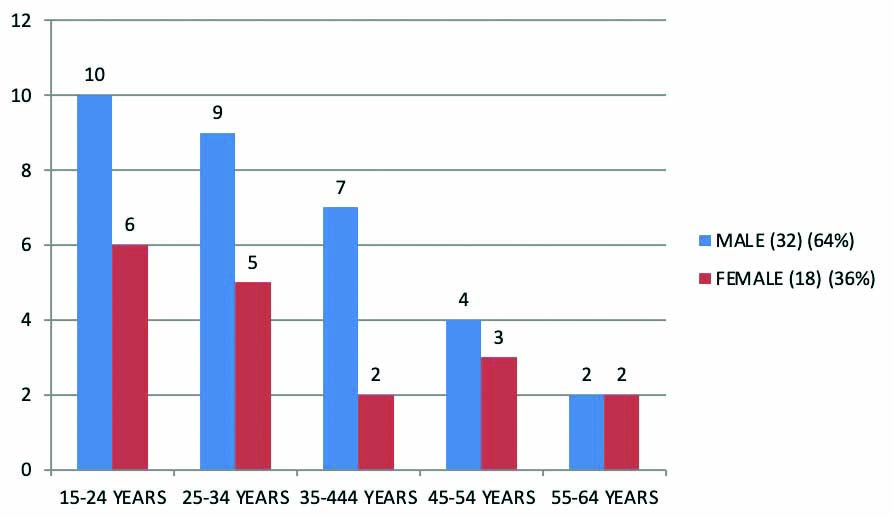
Among 50 cases in NNAC group, 16 cases (32%) were present in 15-24 years age group, 14 cases (28%) were in 25-34 years age group, nine cases were in 35-44 years age group, seven cases were in 45-54 years age group and four cases were more than 54 years. Most of the cases were present in 15-24 years age group [Table/Fig-3].
Age distribution in NNAC group.
NNAC: Non N-Acetylcysteine
The mean age of the patients for NAC group was 33.3±12.8 years and for NNAC group was 33.2±12.1 years with majority being males in both the groups.
[Table/Fig-4] shows that the comparison in NAC group of mean serum creatinine on day 1, day 3 and day 5 was found to be insignificant (p=0.810) whereas the comparison of mean urine output on day 1, day 3 and day 5 was statistically significant (p <0.001).
Mean serum creatinine (mg/dL) and urine output (mL/24 hr) in NAC group.
| Parameters | Day 1 | Day 3 | Day 5 | p-value |
|---|
| Mean creatinine (mg/dL) | 2.42±0.82 | 2.53±1.0 | 2.6±1.7 | 0.810 |
| Mean 24 hr urine output (mL) | 503.0±153.3 | 611±185.0 | 788±250.6 | <0.001* |
NAC: N-Acetylcysteine, *Statistically significant, ANOVA test
[Table/Fig-5] shows that the comparison in NNAC group of mean serum creatinine on day 1, day 3 and day 5 was found to be insignificant (p=0.354) whereas the comparison of mean urine output on day 1, day 3 and day 5 was significant (p<0.001).
Mean serum creatinine (mg/dL) and urine output (mL/24 hr) in NNAC group.
| Parameters | Day 1 | Day 3 | Day 5 | p-value |
|---|
| Mean creatinine (mg/dL) | 2.4±0.82 | 2.6±1.1 | 2.8±1.8 | 0.354 |
| Mean 24 hr urine output (mL) | 517±158.6 | 672±211.7 | 798±303.9 | <0.001* |
NNAC: Non N-Acetylcysteine
*Statistically significant, ANOVA test
[Table/Fig-6] shows the mean and standard deviation among both NAC and NNAC groups. The mean duration of fever at the time of presentation was found to be five days in this study. The mean of total serum bilirubin in mg/dL was found to be 1.7±0.9 in NAC group and 1.7±0.8 in NNAC group. The study found that in most of the cases the more severe the AKI, the more increase in the total serum bilirubin. Mean Hb level in gm/dL was found to be 10.2±1.3 and 10.3±1.28 in NAC group and NNAC group, respectively. Most cases of anaemia were present in those whose serum creatinine was raised, indicating some kind of relationship between Red Blood Cells (RBCs) destruction and renal impairment, perhaps a common mechanism [32]. The p-value was calculated in order to determine the significance of the difference noted between the two groups.
Comparisons of parameters between NAC AND NNAC group.
| Parameters | NAC | NNAC | p-value |
|---|
| Weight (kilogram) | 50.8±7.0 | 53.0±7.3 | 0.085 |
| Total serum bilirubin (mg/dL) | 1.7±0.9 | 1.7±0.8 | 0.852 |
| Haemoglobin (g/dL) | 10.2±1.3 | 10.3±1.28 | 0.917 |
| Serum Creatinine Day 1 (mg/dL) | 2.42±0.82 | 2.4±0.82 | 0.997 |
| Serum Creatinine Day 3 (mg/dL) | 2.53±1.0 | 2.6±1.1 | 0.634 |
| Serum Creatinine Day 5 (mg/dL) | 2.6±1.7 | 2.8±1.8 | 0.553 |
| Urine Output Day 1 (mL) | 503±153.3 | 517±158.6 | 0.751 |
| Urine Output Day 3 (mL) | 611±185 | 672±211.7 | 0.204 |
| Urine Output Day 5 (mL) | 788±250.6 | 798±303.9 | 0.980 |
| CYSCD1 (mg/dL) | 2.236±0.78 | 2.198±0.74 | 0.864 |
| Fever (days) | 5.1±1.0 | 5.12±0.96 | 0.854 |
CYSCD1- Serum Cystatin-C on day one,
NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine
*Statistically significant, Student t-test
[Table/Fig-7] shows that out of 50 cases who were given NAC 600 mg twice daily for five days, 28 (56%) cases were improved in AKI on day 5 as compared to day 1 of the study in NAC group.
Outcome of AKI among NAC and NNAC group with increment and decrement of serum creatinine.
| Cases | Improved | Deterioration | p-value |
|---|
| NAC group | 28 (56%) | 22 (44%) | χ2, 0.04; p=0.841 |
| NNAC group | 26 (52%) | 24 (48%) |
NAC; N-Acetylcysteine; NNAC: Non N-Acetylcysteine
*Statistically significant; Chi square test
Similarly, out of 50 cases who were not given NAC, 26 (52%) cases were improved in AKI on day 5 as compared to day 1 of the study in NNAC group.
There was no difference in patients showing improvement in AKI after NAC therapy compared to patients with NNAC (χ2, 0.04; p=0.841).
[Table/Fig-8] shows that out of 50 cases in NAC group, seven cases were in KDIGO STAGE-3 (AKI) on day 1 and on day 5 it was observed that 12 cases were in KDIGO STAGE-3 (AKI). In NNAC group, out of 50 patients seven patients were in KDIGO STAGE-3 (AKI) on day 1 and on day 5 the number of patients in KDIGO STAGE-3 (AKI) were increased to 11.
Impact of NAC on KDIGO stage-3 (AKI).
| No. of patients in KDIGO stage-3 | NAC group | NNAC group |
|---|
| On day 1 | 7 | 7 |
| On day 5 | 12 | 11 |
KDIGO: Kidney disease improving global outcomes; NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine
Out of 50 cases in NAC group, 10 cases were oliguric on day 1 of the study. Similarly, in NNAC group out of 50 cases, only 11 cases were oliguric on day 1 of the study [Table/Fig-9].
Oliguric cases on day 1 in NAC and NNAC group.
| AKI with urine output on day 1 | NAC group (n=50) | NNAC group (n=50) | p-value |
|---|
| Oliguria | 10 (20%) | 11 (22%) | χ2, 0.06; p= 0.806 |
| Non-oliguria | 40 (80%) | 39 (78%) |
AKI: Acute kidney injury; NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine; *Statistically significant, Chi-square test
Discussion
The study gave NAC in falciparum malaria after the renal insult for further prevention of AKI. It has been shown to have a protective effect on CI-AKI when NAC was administered before the onset of renal insult [33]. The emergence of AKI as an important clinical manifestation of severe P.falciparum malaria in adults has also been described in earlier studies from Vietnam and from different parts of India [34-37]. The mean age of the patients for NAC group was 33.3±12.8 years and for NNAC group was 33.2±12.1 years with majority being males in both the groups. According to WHO 2007, given equal exposure, adult men and women are similarly vulnerable to malaria infection, with the exception of pregnant women who are at bigger risk of severe malaria in major endemic areas [38]. In this study, most of the cases were present in 15-34 years age group in both NAC and NNAC group. The above facts suggest that the younger generation was more susceptible to malarial infection, as well as more prone to severity and death arising out of the disease. Similar findings were found in a study conducted by Patriani D et al., [39].
Fever for treatment duration of more than four days is associated with increased risk of AKI [40,41]. Inadequate treatment with artemisin derivative and associated muscle injury due to intramuscular artether may precipitate AKI. High parasitic count and hypotension are also important risk factors for the development of AKI [40,42]. The mean serum cystatin-C was found to be 2.236±0.78 mg/dL in NAC group and 2.198±0.74 mg/dL in NNAC group on day 1 of the study. The sensitivity and specificity of serum cystatin-C as a biomarker for AKI is better than serum creatinine [43,44]. Hence, serum cystatin-C analysis in all cases on day 1 was performed. KDIGO staging on day 1 was difficult as most of the cases the basal serum creatinine was not known. But if serum creatinine was ≥4 mg/dL then it was designated as stage-3 AKI according to KDIGO criteria excluding CKD [31]. So, KDIGO stage-3 cases on day 1 and on day 5 in both the groups was taken. It was found that KDIGO stage-3 cases were increased on day 5 as compared to day 1. This would be due to natural course of falciparum malarial AKI [45].
A sizable bulk of cases included in this study had falciparum malaria with mild to moderate AKI. The possibility could be due to the cases with severe AKI needing haemodialysis were excluded from the study. It was found that mortality in NAC group is less than NNAC group. The effect of NAC on the incidence of CI-AKI is quite variable. To date, out of several meta-analyses that have been published found a net benefit for NAC in the prevention of CI-AKI [46].
The cases remained in this study were those whose urine output was normal or good and serum creatinine were not so much high and there was gradual improvement and deterioration in serum creatinine from day 1 to day 5. It was found that improvement in AKI cases was 56% in NAC group as compared to 52% in NNAC group. Though there was more improvement in NAC group but statistically it was found to be non-significant (χ2, 0.04; p=0.841). NAC might have beneficial effect in falciparum malarial AKI. But it needs large scale trial.
Limitation(s)
As in most of the cases no baseline serum creatinine was available, it was difficult to establish whether the raised serum creatinine level was due to AKI or underlying CKD on first day of the study. There was lack of availability of baseline serum creatinine and precise hour to hour monitoring of urine output as most lay in general wards. So, it was difficult to categorise AKI (stage-1 and stage-2) according to KDIGO criteria. The study have not analysed the effect of parasitemia with the severity of AKI in P.falciparum malaria. Number of deaths could have been underestimated due to the fact that as the condition deteriorated the patient was taken away to other medical centers, while some others took the sick patients back home due to lack of monetary funding. NAC was given through oral route in a low dose and for short duration (five days). As oral bioavailability of NAC is low and also, the authors administered it for a short duration, its efficacy seemed to be very less.
Conclusion(s)
It was found that oral NAC was safe, cheap and well-tolerated in patients of falciparum malaria with AKI and no adverse effect were noted. With the unavailabity of serum cystatin-C; KDIGO criteria may be used for diagnosis of AKI routinely. In NAC group improvement of falciparum malarial AKI after five days was found to be little bit more as compared to the NNAC group though it did not reach statistically significant difference. So, the overall benefit of NAC was not consistent or overwhelming. The scientific justification for its use in severe malaria is stout and needs further study in a great level, double blind randomised clinical trial. Therefore, a more comprehensive study with large population with high dose of NAC and a longer period of study would be more informative.
Author’s contribution
Dr. Gurupada Das, Department of Neurology, is the primary investigator, made the data collection. Dr. P. C. Karua, Associate Professor, Department of Medicine made the design of the study, redaction of the manuscript, analysis of data and data interpretation. Dr. Rama Chandra Sethy is the corresponding author. Dr. Bibhu Prasad Behera and Dr. Rama Chandra Sethy made the redaction of the manuscript, statistic study, analysis of data and data interpretation.
NAC: N-Acetylcysteine, *Statistically significant, ANOVA test
NNAC: Non N-Acetylcysteine
*Statistically significant, ANOVA test
CYSCD1- Serum Cystatin-C on day one,
NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine
*Statistically significant, Student t-test
NAC; N-Acetylcysteine; NNAC: Non N-Acetylcysteine
*Statistically significant; Chi square test
KDIGO: Kidney disease improving global outcomes; NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine
AKI: Acute kidney injury; NAC: N-Acetylcysteine; NNAC: Non N-Acetylcysteine; *Statistically significant, Chi-square test