Tuberculosis is a major global health problem. Worldwide, TB is one of the top 10 causes of deaths. Globally, the annual incidence of TB estimates about 10.0 million people of which 2.7 million cases are reported from India [1]. As there is no single test that can be used to detect TB in developing countries like India, a novel cost-effective test is required for its diagnosis. The conventional diagnostic modalities have many limitations as it is time consuming, cumbersome and lacks sensitivity. This often delays the early diagnosis and treatment of TB [2].
EPTB affects many organs or sites and is caused by MTB. The various forms of EPTB are lymph node, central nervous system, pleural, abdominal, bone and joint, pericardial, urogenital, cutaneous and ocular. While EPTB accounts for 15-20% of all TB cases in HIV negative patients, its burden in HIV positive patients is high and it is estimated to be 40-50% [3]. Due to the lack of rapid and cost-effective testing methods, there has been a delay in the reduction of TB-related morbidity and mortality. In developing countries, where 95% of new TB cases and death occurs due to TB, smear microscopy remains the most common and often the only test available that detects only 45% of TB infections [4,5].
Smear microscopy by ZN technique is the cornerstone for the diagnosis of TB in resource-limited settings but it has relatively moderate (80%) sensitivity and a poor PPV [6]. Culture is the “gold standard” for final determination, and also permits drug susceptibility testing. However, it takes 2-6 weeks and delays the initiation of treatment [7].
Nucleic Acid Amplification Testing (NAAT) on direct samples is considered to be rapid than culture and have high turn around time as specimens are being sent to distant laboratories. PCR testing is expensive which makes it inaccessible for the patients in TB-endemic countries [2]. The commonest target used in PCR test is IS6110 and this has good sensitivity and specificity for the diagnosis of pulmonary and EPTB [8].
The miniaturised forms of PCR tests have the advantages of a reduction in the cost of instruments and faster turn around times in poor resource settings. The micro-PCR devices have the added advantages of better diagnostic sensitivity and portability. They are widely used in India and other South-East Asian countries [9]. GeneXpert was endorsed by the World Health Organisation (WHO) to be used in India as part of the National TB control programme [10]. Cartridge Based Nucleic Acid Amplification Test (CBNAAT) is a test which detects TB bacilli and also screens for rifampicin drug resistance [11]. The assay is optimised and used specifically for case detection of pulmonary TB in the public sector in India. The sensitivity of the assay for EPTB is highly variable, ranging from 25%-96% in different studies [12-15]. Lower sensitivities are reported for samples like Cerebrospinal Fluid (CSF) and other sterile site body fluids [16].
Especially in India, there is a need for a more cost-effective rapid method for diagnosis. Truenat is a novel method, which is a point-of-care, battery operated and chip-based RT-PCR micro device for the detection of MTB in clinical samples. It can be used in low-infrastructure settings to give rapid diagnosis with a very small amount of sample. In Truenat MTB ‘detected’ samples, the presence of rifampicin drug resistance can be tested with a second chip as a follow-on test. Truenat MTB test has high sensitivity and specificity for the early diagnosis of pulmonary and EPTB when compared to GeneXpert [17]. However, there is no study where it was validated against culture methods with extrapulmonary specimens. Moreover, since Pathanamthitta district of Kerala has one of the lowest prevalence of TB in the country, the PPV is bound to reduce too. Therefore, it is important to estimate the ability of Truenat MTB to diagnose TB correctly, especially EPTB which has even lower prevalence. The present study was aimed to evaluate Truenat MTB test in comparison with microscopy and culture for the diagnosis of EPTB.
Materials and Methods
This was a prospective cross-sectional study which was done in a tertiary care centre in central Kerala, India during the period from January 2019 to December 2019. Total 248 extrapulmonary clinical samples were obtained during this period. This study was conducted after getting clearance from Institutional Ethical Committee (IEC) with registration number ECR/1098/Inst/KL/2018.
Inclusion criteria: All presumptive EPTB cases irrespective of age, previous history of Anti-Tubercular Treatment (ATT) and whose treatment failed at the end of their most recent course of treatment were included in the study. The different forms of EPTB includes lymph node TB, pleural TB, abdominal TB, central nervous system TB, pericardial TB, spinal TB, bone and joint TB, genitourinary TB, ocular TB and cutaneous TB [18].
Exclusion criteria: Patients who were on ATT were excluded from the study.
Diagnosis of EPTB: Any clinical sample in which MTB was detected by Truenat MTB test or positive culture/microscopy was considered as a microbiologically proven case of EPTB. Any sample which was positive with culture/microscopy and if MTB was not detected by Truenat was evaluated for presence of Non-Tuberculous Mycobacteria (NTM) by culture [10,19].
Processing of the clinical specimens: Various specimens like tissue biopsies, pleural fluid, ascitic fluid, peritoneal fluid, urine, synovial fluid, CSF, bone specimens, liver aspirate and pus from cold abscess/deep sites were obtained from suspected cases after getting informed consent from the patients. All the fluid specimens were centrifuged at 5000 rpm (revolutions per minute) for five minutes in a sterile tube. The tissue specimens are washed with sterile water and they are homogenised by using mortar and pestle or a micro pestle.
Truenat MTB plus test: Truenat MTB Plus test was performed as per manufacturer’s instructions [20,21].
Sample processing procedure: All the samples were treated as per Molbio EPTB sample pre-treatment protocol. After discarding the supernatant of the centrifuged specimens, 0.5 mL of the sediment was transferred to Lysis buffer tube. The homogenised tissue sample and pus aspirate were treated with liquefaction buffer for five minutes and then transferred to Lysis buffer tube. The tube was then vortexed for five minutes.
Extraction procedure of Trueprep auto kit: The extraction of Deoxyribonucleic Acid (DNA) from the samples was done using Trueprep AUTO Universal Cartridge Based Sample Prep kit and device. The pre-treated sample was transferred to the sample chamber of the cartridge and was placed in the device. The entire elute was aspirated out from the elute chamber into the Elute Collection Tube (ECT).
Truenat MTB plus real time PCR: The 6 μL of purified DNA from ECT was transferred to microtube containing freeze dried PCR reagents. It was then added to the Truenat MTB microchip containing lyophilised mastermix and the real-time PCR was done using a pre- programmed profile on Truelab Analyser. Results obtained using the Real-time PCR were reported as ‘not detected’ for negative samples or ‘detected with number of Colony Forming Units per milliliter (CFU/mL)’ for positive samples. The Limit of Detection (LoD) in Truenat MTB test was 100 CFU/mL and a follow on test for rifampicin resistance was planned to be performed using Truenat MTB-RIF Dx in those positive samples with more than 10,000 CFU/mL.
Conventional culture and microscopy: The samples after digestion and concentration were processed for culture in Lowenstein Jensen media and for smear microscopy as per standard protocols [22,23]. The culture bottles were examined daily for the initial one week and thereafter weekly once for eight weeks. Ziehl-Neelsen staining was performed and observed for the presence of acid fast bacilli [Table/Fig-1].
Acid fast bacilli (arrow) in Ziehl-Neelsen staining (100x magnification) of pus aspirate sample.
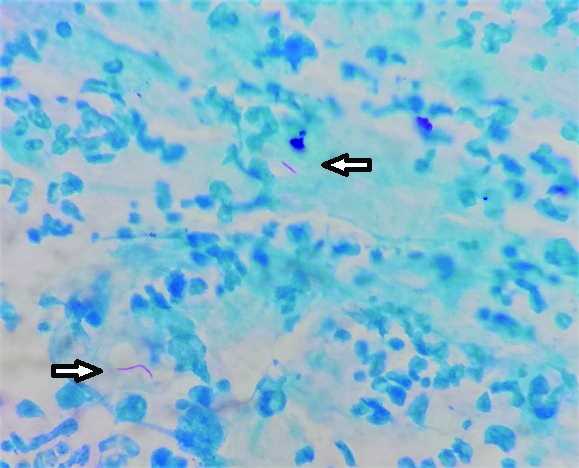
Out of 248 samples received, all were subjected to Truenat MTB test. Due to insufficiency of samples and unforeseen reasons, only 107 samples were proceeded for culture and 179 samples for ZN staining. The results of Truenat, microscopy and culture were analysed. Observed percentage of agreement, sensitivity, specificity, PPV and NPV of Truenat were evaluated over culture and microscopy.
Statistical Analysis
Comparisons of sensitivity, specificity, NPV, PPV and observed agreement of Truenat MTB test with other tests were applied. Data was presented using summary statistics with 95% CI. Since this study is first of its kind, the estimation of sample size was overwhelming without prior knowledge of the expected test discordance and may be an underestimate of the sample size required.
Results
A total of 248 suspected cases were studied over a period of one year from January 2019 to December 2019. Samples were obtained from various departments like Surgery, Urology, Medicine, Orthopaedics, Paediatrics, Neurosurgery, Gastroenterology, Pulmonary Medicine and Gynaecology [Table/Fig-2].
Distribution of samples from different departments.
| Department | Number of samples (%) |
|---|
| General medicine | 82 (33.1) |
| General surgery | 55 (22.2) |
| Urology | 52 (21) |
| Gastroenterology | 26 (10.5) |
| Pulmonary medicine | 10 (4) |
| Orthopaedics | 10 (4) |
| Neurosurgery | 9 (3.6) |
| Paediatrics | 3 (1.2) |
| Obstetrics and gynaecology | 1 (0.4) |
| Total | 248 |
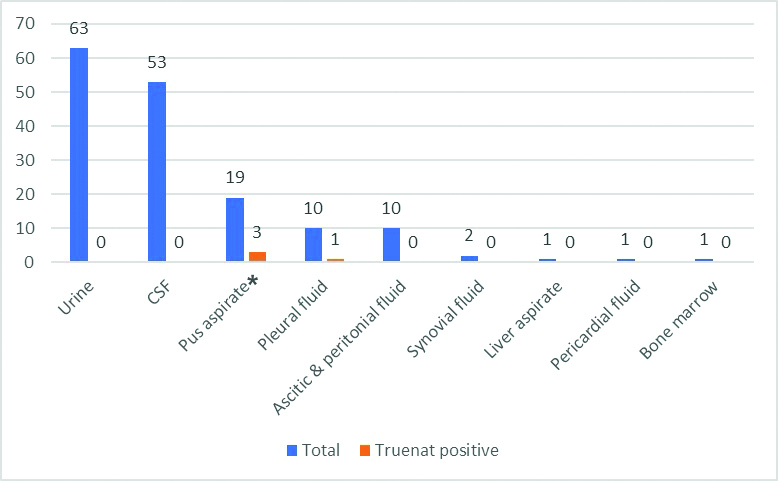
There was an almost equal distribution of males and females among the study population with 126 males (50.8%) and 122 females (49.2%). Maximum number of suspected EPTB patients was in the age group of 50-59 and 60-69 age category (42.3%). Age wise distribution of samples is mentioned in [Table/Fig-3]. A total of 160 (64.5%) fluid samples and 88 (35.5%) tissue sample were received. Out of the different fluid samples, urine (63, 39.4%), CSF (53, 33.1%) and pus aspirate (19, 11.9%) were the majority. Of the tissue samples received, lymph node (36, 40.9%), intestinal biopsy (24, 27.3%) and skeletal (16, 18.2%) constituted the majority. [Table/Fig-4,5] shows the different fluid and tissue specimens which were studied.
Age wise distribution of samples.
| Age group (years) | Number of samples | Number of Truenat MTB positive samples | Number of NTM positive samples |
|---|
| 0-9 | 3 | -- | -- |
| 10-19 | 14 | -- | -- |
| 20-29 | 27 | 3 | -- |
| 30-39 | 29 | 2 | -- |
| 40-49 | 40 | -- | -- |
| 50-59 | 52 | 3 | -- |
| 60-69 | 53 | -- | 1 |
| 70-79 | 20 | 1 | -- |
| 80-89 | 10 | -- | -- |
| Total | 248 | 9 | 1 |
MTB: Mycobacterium tuberculosis; NTM: Non tuberculous mycobacteria
Distribution of fluid specimen types and its Truenat positivity.
CSF: Cerebrospinal fluid; *Three aspirated pus samples which were positive for MTB was from lymph node abscesses.
Distribution of tissue/solid specimen types and its Truenat positivity.
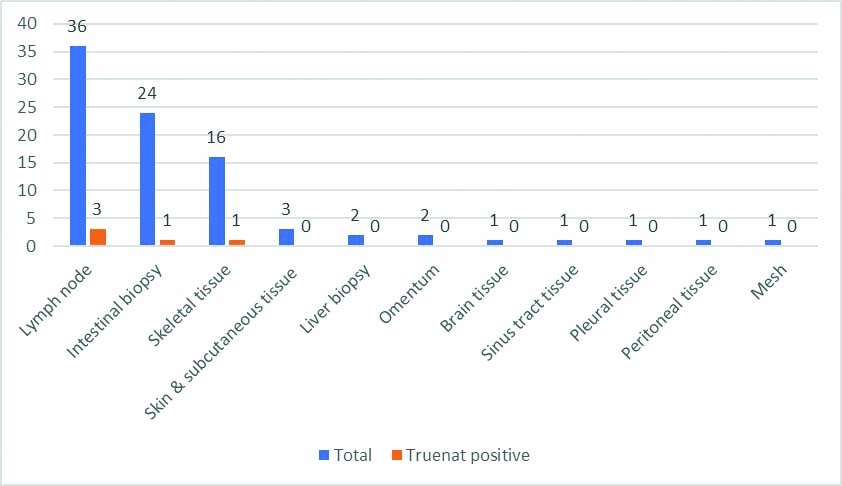
Out of the 248 samples tested, 9 (3.6%) were positive for MTB by Truenat. Out of the different positive samples, three were pus aspirates (33.3%), three were lymph node tissue (33.3%) and one each were pleural fluid, intestinal biopsy and skeletal tissue (11.1% each) [Table/Fig-4,5]. All the positive aspirated pus was from lymph node cold abscesses. In the present study, lymph node TB was the predominant type of EPTB (6, 66.6%). Maximum number of positive cases were in the age group category 20-29 (33.3%) and 50-59 (33.3%).
Culture in conventional solid media (LJ) was performed with 106 samples, out of which MTB was isolated in four samples. All culture positive samples were positive for MTB by Truenat also. Apart from these, one sample (infected mesh after herniorrhaphy) which was culture positive for NTM was negative when tested with Truenat MTB. Out of the 179 samples subjected to ZN staining, acid fast bacilli were seen in four samples. Of the smear positive samples, one was later on confirmed with culture as NTM and was excluded from statistical analysis, so sample for ZN was 178.
The effectiveness of Truenat as a screening test for EPTB was assessed by comparing the results with culture as the gold standard and with microscopy. The sensitivity, specificity, PPV, NPV and observed agreement of Truenat with culture and microscopy are summarised in [Table/Fig-6].
Comparison of Truenat with culture and microscopy.
| Tests | Results | Truenat | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Observed agreement (%) |
|---|
| Positive (n) | Negative (n) |
|---|
| Culture (n=106)‡ | Positive (n) | 4 | 0 | 100 | 95.1 | 44.4 | 100 | 95.3 |
| Negative (n) | 5 | 97 |
| Microscopy (n=178)‡ | Positive (n) | 3 | 0 | 100 | 96.6 | 33.3 | 100 | 96.6 |
| Negative (n) | 6 | 169 |
*Comparisons were made using summary statistics with 95% CI.
‡Out of 179 samples for microscopy, one was negative with Truenat which was confirmed as NTM with culture. Hence, comparison was performed with 178 samples for microscopy and 106 samples for culture
Discussion
The present study had a high concordance for Truenat against culture and microscopy with high sensitivity, specificity, PPV and NPV showing the effectiveness of this innovative tool from India. But unlike other studies which involved pulmonary samples, this study focused on extrapulmonary samples which is now showing an increasing proportional trend [16,20]. This study is the first of its kind to evaluate the effectiveness of Truenat for extrapulmonary specimens as there is a scarcity of literature on the effectiveness of Truenat in diagnosis of EPTB [24].
This study was conducted with a total of 248 samples of presumptive TB cases received from various clinical departments to the Microbiology and TB unit of a tertiary care teaching institution in central Kerala, India. EPTB was seen more in females (6 out of 9 positives) which are in par with another study [25]. The global reports on TB by WHO found a higher incidence of the disease among males [26]. TB affects mostly the adult age groups in the developing world and this study is no different. Maximum number of positive cases (three each) was in the age group of 20-29 and 50-59 category. Although the highest burden of TB is among adult men, people of all age groups are affected [25]. Lymph node TB was the predominant type in present study (6, 66.6%) followed by intestinal, pleural and skeletal TB (1 each, 11.1%). The most common type of EPTB was lymph node TB followed by pleural TB in other studies [25,27]. Endometrium was the commonest sample type in a study on the diagnosis of EPTB by Truelab MicroPCR by Ranjana H and Sadhna S [24].
With culture as gold standard test, Truenat had a sensitivity of 100% and specificity of 95.1%. When compared with microscopy, Truenat test had 100% sensitivity and 96.6% specificity. In a similar study conducted by Nikam C et al., for evaluation of Truenat using sputum samples, they found a sensitivity value of 100% and a specificity value of 43.98% for Truenat over microscopy, whereas for Truenat over culture they obtained a sensitivity value of 94.70% and a specificity value of 52.85% [17]. In another study conducted by Nikam C et al., it was reported that the Truenat MTB was able to detect TB rapidly with good sensitivity in comparison with a Composite Reference Standard (CRS) [20]. Present study results were in concordance with this study, though there was higher specificity also. Both the above studies evaluated the effectiveness of Truenat MTB using pulmonary samples unlike present study which is presumed to be the first of its kind in EPTB.
One sample which was Truenat negative was positive in smear microscopy and NTM was isolated in culture. For the diagnosis of PTB and EPTB, culture and microscopy are also of value along with the molecular tests, as Truenat detects only MTB. This was comparable to another study which compared culture, microscopy and GeneXpert [28]. For the detection of both MTB and NTM, multiplex Real Time PCR should be used [29].
The load of bacilli required to obtain a positive value for rifampicin resistance is nearly 104 bacilli however this study was conducted with extrapulmonary specimens where the bacilli load was even less so the authors were unable to perform rifampicin resistance using Truenat in all positive cases. In present study, the bacterial load of positive samples ranged from 100 to 1000. However, in the three positive samples screened, rifampicin resistance was not detected. Rifampicin sensitivity testing in EPTB has to be evaluated in larger sample sizes and the need to depend on alternate methods has to be studied. In a study by Vijayalakshmi J et al., on evaluation of Truenat with pulmonary samples, Rifampicin drug sensitivity was tested with Truenat MTB/RIF micro PCR chip and resistance was detected in 19% of the samples [21].
In the present study, the Truenat MTB test allows detection of TB in approximately 2 to 3 hours and can be utilised in near-care settings to provide quick and accurate diagnosis. Truenat MTB is a good point-of-care, linkage-to-care and treatment test which is cost effective according to Lee DJ et al., study [30]. Truenat MTB test has a higher sensitivity than other conventional diagnostic tests like smear microscopy or culture for MTB. The present study stresses the importance of this new tool, which is indigenous, economical and convenient to use in a low resource setting like India. This study paves and opens windows for larger studies for replacing other molecular diagnostic tests.
Limitation(s)
Authors have compared the effectiveness of Truenat with microscopy and conventional culture for a period of one year. Since the prevalence of EPTB is low, larger studies are required to compare its effectiveness with GeneXpert and MGIT tests to estimate the performance of Truenat MTB. Due to insufficiency of samples, the preference was given to PCR test and not all samples were subjected for microscopy and culture. Rifampicin resistance can be detected only when the bacilli load is more than 104 CFU/mL. However, most of the samples that was received were with low bacterial load and therefore, rifampicin resistance could not be assessed.
Conclusion(s)
Truenat MTB test has high sensitivity and specificity for the case detection of EPTB with a testing time of less than three hours. As the NPV is high, this will be an ideal test for screening of TB. In the current study, Truenat assay showed a high concordance with other studies on molecular diagnostic tests. Thus, this assay might be a potential, accurate, and rapid method for the detection of EPTB cases in low resource settings.
MTB: Mycobacterium tuberculosis; NTM: Non tuberculous mycobacteria
*Comparisons were made using summary statistics with 95% CI.
‡Out of 179 samples for microscopy, one was negative with Truenat which was confirmed as NTM with culture. Hence, comparison was performed with 178 samples for microscopy and 106 samples for culture