Liver as a vital metabolic organ, is almost difficult to replace by any substitute methods, as it is the main organ of metabolism and energy production. Hence, it is a susceptible target for injury from medications and chemicals. The most frequent reason for the failure of new drug molecules during various phases of new drug development or for the withdrawal of drugs is hepatotoxicity [1]. Hepatotoxicity can be predicted by assessing the level of mitochondrial damage, oxidative stress and intracellular GSH by high content cellular imaging in primary human hepatocyte cultures [2]. Cysteine is a rate limiting step for GSH synthesis and hence, methionine metabolism via S-adenosyl-methionine (SAM) and trans-sulfuration is very important in regulating GSH levels in the liver; the effects of single oral dose DL-Methionine were studied by the researcher and part of the same has already been carried out [3].
Oxidative stress is one of the major causes of drug-induced liver diseases. Synthesis of GSH, the most abundant mammalian antioxidant, is regulated at the substrate level by cysteine, which is synthesised from homocysteine via the trans-sulfuration pathway [4]. GSH is an important antioxidant and cofactor for GSH S-transferase conjugation. GSH synthesis is catalysed by Glutamate Cysteine Ligase (GCL) [5]. N-acetyl derivative of the naturally occurring amino acid, L-cysteine i.e., oral NAC is deacetylated into L-cysteine which is a direct precursor in the synthesis of intracellular GSH, also a good source of sulfhydryl containing antioxidant; acts directly as free radical scavenger. Its antioxidant action is believed to originate from its ability to stimulate GSH synthesising level and scavenging Reactive Oxygen Species (ROS) and nitrogen species [6]. The depleted GSH levels are restored by hepatoprotective agent, acetylcysteine. Acetylcysteine can also act as an alternate substrate for conjugation with the toxic reactive metabolite, also may act either by reducing the metabolite to the parent compound and/or by supplying-SH group for conjugation; or may directly inactivate the toxic metabolite [6].
If the liver is damaged or injured, the liver enzymes spill into the blood, causing elevated liver enzyme levels. The levels of the liver enzymes like transaminases, alkaline phosphatase, γ-glutamyl transpeptidase, in the blood can be measured to know the normal functioning of liver. These enzymes help in detecting injury to hepatocytes. Hence, through Liver Function Tests (LFTs) and their most likely interpretations or analyses of certain enzyme activities in blood serum, gives valuable diagnostic information for a number of disease conditions, including liver injury. Level of liver injury can be investigated through the determination of LFTs, mainly serum alanine aminotransferase (ALT) which is regarded to be a significant, sensitive and a specific preclinical and clinical biomarker of liver damage [7,8]. NAC was introduced in the 1970’s and still is the current clinical treatment for acetaminophen overdose [9]. There is a growing need to research into liver diseases and hepatoprotective remedies as shown by Orhan DD et al., and according to researchers, it was documented for the need of further research in this area [10].
Hence, the present study was aimed to demonstrate the effect of orally administered NAC, as a single oral dose on albino rats.
Materials and Methods
The present research study is of experimental study design, interventional type which includes the use of healthy albino rats that were housed in animal house, SBKS Medical Institute and Research Centre. This study was conducted for a duration of one year. This included acquiring the chemicals, drugs and other necessary logistics. After the experimental design was finalised, histopathology slides and biochemical parameters were studied, as a part of result. The experimental procedure was conducted from May 2015 till August 2015, i.e. dissection, administration of drugs and collection of specimens.
Ethical approval: The protocol (no. SVU/PH/IAEC/25-04-2009/01) was accepted and approved by the Institutional Animal Ethics Committee (IAEC), (CPCSEA Reg. No.: 947/PO/Re/S/06/CPCSEA, Dated 30-06-2006) of SBKS Medical Institute and Research Centre, Sumandeep Vidyapeeth Deemed to be University, Piparia, Vadodara; which is registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forest and Climate change, Government of India, New Delhi.
Materials: The drug and chemicals used were NAC 10 gm extrapure from Aatur Instru Chem, Vadodara. Other materials included the Diagnostic kit reagents for the estimation of LFTs, distilled water and ether. The chemicals used were 10% formalin, xylene, Haemotoxylin and Eosin (H&E) stains, for preparation of histopathology slides.
To evaluate the levels of liver enzymes, Serum Glutamic-Pyruvic Transaminase (SGPT), Serum Glutamic-Oxaloacetic Aminotransferases (SGOT), Serum Alkaline Phosphatase, Serum bilirubin- Direct and Indirect Bilirubin, Total Bilirubin; Serum Gamma-Glutamyl Transpeptidase (GGTP) the diagnostic kit reagents (Erba Diagnostics, Manheim) was used. Standard Erba estimation kit was used by using auto analyser (Erba, Chem7 Germany). Standard procedure as specified in the kit literature was followed.
Animals and Experimental Design
All studies were conducted in young healthy albino rats of either sex weighing 150-400 gm body weight which were separated before starting the study. Before initiation of any procedures, they were provided housing in polypropylene rat cages, acclimatised and were given free access to food and purified drinking water ad libitum and rice husk used as bedding. Animals were kept under controlled environmental conditions. They were maintained under a controlled ambient temperature (24°±2°C), relative humidity (55%±5%), and in a 12-hour light/dark rhythm, respectively 7am to 7pm light; throughout the experiment.
Dose and Experimental Groups
As depicted in [Table/Fig-1] young healthy albino rats were randomly divided into two groups for the experimental study; Group I vehicle-treated and Group II NAC-treated [11,12]. Albino rats (n=6) in Group I were administered distilled water (10 mL/kg body weight) orally by intragastric cannula, was considered as control group. The albino rats (n=6) that were administered NAC as a single dose oral administration by intragastric cannula at dose of 450 mg/kg body weight [11] in each rat, to demonstrate their effects of oral administration was considered as Group II.
Experimental groups and study protocol [11,12].
| Sr. No. | Groups | n | Treatment | Duration of treatment |
|---|
| 1. | Group I Control | 6 | Distilled water10 mL/kg body weight | 24 hours overnight fasting |
| 2. | Group II NAC | 6 | N-Acetylcysteine450 mg/kg body weight | 24 hours overnight fasting |
A total of 12 albino rats were separated for overnight fasting, six in each group. Group I was the control group and each rat of this group was given distilled water 10 mL/kg orally by gastric tube. Each rat in Group II was given NAC 450 mg/kg based on the body weight as a single oral dose [11]. As per the study done by researcher Ferguson HC, the rats were administered the drug solutions which were freshly prepared before use with the volume of 10 mL/kg, hence the volume administered was maintained constant at 10 mL/kg of the body weight [12]. All the drugs and control vehicle were administered by per oral (po) and the volume administered was maintained constant in all the albino rats at 10 mL/kg body weight.
After 24 hours of post-treatment with NAC; under light ether anesthesia; whole blood was collected in labelled glass collecting tubes from retro-orbital plexus of eye with the help of glass capillary tube, for estimation of haemato-biochemical parameters in serum. Serum was stored at -20°C until analysed and were assessed to determine the extent of liver injury at the end of 24 hours of exposure of the drug. Serum was separated immediately through centrifugation at 3000 rpm for 10 minutes for the determination of liver enzymes, Alanine Amino Transferase, Aspartate Amino Transferase, Alkaline Phosphatase, Gamma-Glutamyl Transpeptidase (GGTP) or Gamma-Glutamyl Transferase (GGT), and Total Bilirubin [13-17].
Histopathological Examination
From the experimental group of albino rats, after collection of serum, liver from each albino rat was immediately dissected out, isolated, and washed with normal saline in glass petridish and preserved in 10% formalin for fixation for histopathological studies in separately labelled specimen collection jars.
The liver samples which were fixed in 10% formalin, were processed for paraffin embedding. The sections were taken at about 4-6 μm thickness and were stained with H&E. The liver sections were microscopically examined for the study of histopathological changes under 40x or 100x magnifications [18].
Statistical Analysis
The experimental data collected were analysed using a statistical programme using computer-based statistical software SPSS version 21.0; and the results were expressed as Mean±SEM, in terms of International Units Litre-1.
Results
It was observed that, NAC as a single oral dose of 450 mg/kg although caused alteration in the levels of liver enzymes; the alteration was not statistically significant, which suggests that NAC has no toxic effect on the liver as indicated in [Table/Fig-2]. In the control group, administered with Distilled Water 10 mL/kg the Mean±SEM values of SGPT (IU/L) recorded were 32.83±2.91, for SGOT (IU/L) 126.00±15.07, for Total Serum Bilirubin (μmol/L) were 0.70±0.08, values of ALP (IU/L) were106.17±23.15 and for GGTP (IU/L) were 2.33±0.56, all the values were analysed and were statistically non-significant.
Shows changes following oral administration of NAC on the levels of Liver enzymes in young healthy albino rats.
| Sr. No. | Group (n=6) | Biochemical parameters of LFTs Mean±SEM values |
|---|
| SGPT (IU/L) | SGOT (IU/L) | Total Serum Bilirubin (μmol/L) | ALP (IU/L) | GGTP (IU/L) |
|---|
| 1. | Group II N-Acetylcysteine 450 mg/kg | 39.00±2.70 | 114.67±16.92 | 0.80±0.05 | 194.00±38.72 | 1.47±0.23 |
Histopathological findings in control group:
a) Gross examination: The liver appeared normal with no changes in the texture [Table/Fig-3].
Liver sample from control rat.
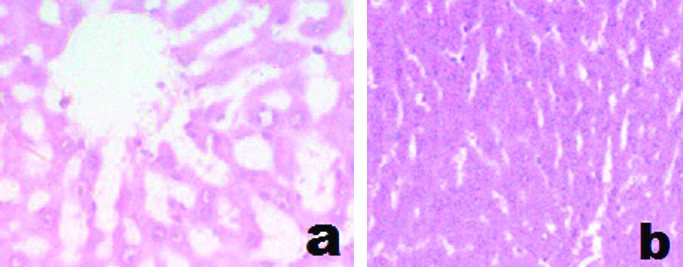
b) Microscopic examination: The light microscopic evaluation of livers, in the control rats, showed normal morphology of hepatic parenchyma [Table/Fig-4a,b].
a) Cross-section of liver tissue from control rat showing central vein (H&E stain x400). b) Cross-section of liver tissue from control rat showing normal hepatocytes (H&E stain x400).
Histopathological findings in single dose oral N-Acetylcysteine (NAC) treated liver:
Following were the observations of the histopathological findings of single dose oral NAC 450 mg/kg body weight, that were compared with the control group.
a) Gross examination: The liver appeared normal as similar to control treated rats [Table/Fig-5].
Liver sample from N-Acetylcysteine (NAC) treated rat.
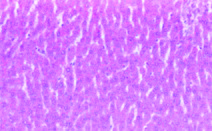
b) Microscopic examination: The liver tissue showed normal morphology and normal hepatic parenchymal cells [Table/Fig-6].
Cross section of liver tissue showing normal hepatocytes in rat treated with N-Acetylcysteine (NAC) (H&E stain x400).
Discussion
The NAC which was first used in the treatment of paracetamol toxicity is a sulfhydryl compound which is supposed to act by replenishing the hepatic stores of GSH. It serves as a prodrug to L-Cysteine, which is a precursor to the antioxidant GSH. The liver is the main metabolic organ and has a central role in drug metabolism. It carries out nearly 500 different functions in human body that are not possible to replace by other alternative methods. Liver is more susceptible target for injury from chemicals and drugs, and disorder of liver function is an important cause of liver injury. Hepatotoxicity has been recorded as one of the most likely complication of majority of the prescribed medications and also most frequent reason for withdrawal of approved medications from the market, or issuing warnings and modifications of drug use. However, it has been documented by several researchers, such as reported by Aithal GP and Day CP that even though numerous medicines related with a significant risk of liver toxicity have been replaced by evidently safer medicaments, adverse liver reactions are still being increasingly documented [19]. Treatment for drug-induced hepatotoxicity generally consists of withdrawing the drug and providing supportive therapy.
In the present research study, NAC is the current clinical treatment for acetaminophen overdose. Its efficacy in the treatment in acetaminophen-overdose has been evident in a recent Cochrane based meta-analytical study by Chiew AL et al., [19,20].
If the liver is damaged or injured, the liver enzymes spill into the blood, causing elevated liver enzyme levels. The levels of the liver enzymes like transaminases, alkaline phosphatase, γ-glutamyl transpeptidase, in the blood can be measured to know the normal functioning of liver. These enzymes help in detecting injury to hepatocytes.
In the present study, it was observed that, in the presence of NAC there occurred no changes in the level of serum enzymes of young healthy albino rats. Moreover, the histopathological examination showed the gross appearance of the liver to be similar to that of the control treated rats, with no change in the texture.
Limitation(s)
The study was conducted in a group of six albino rats, due to reduction in the number of animals per group, based on the principles of the 3 Rs. (Replacement, Reduction and Refinement). The study is done on experimental animals hence, further research can be carried out in different species of animals with other routes of administration also.
Conclusion(s)
NAC as a single oral dose of 450 mg/kg body weight although showed alteration in the haemato-biochemical levels, they were not found to be statistically significant so as to be considered as toxic to liver. Also, the histopathological changes revealed normal appearance and normal morphology of the liver to be similar to that of the control treated rats. Hence, NAC as a single oral dose of 450 mg/kg body weight has no toxic effect on the liver in albino rats.