Introduction
In developing countries, immunosuppressive patients are at greater risk of parasitic infection which may cause morbidity and mortality. Socio-economic and environmental factors including lack of health hygiene in close contact with infected reservoir animal which plays an important role.
Aim
To determine the prevalence of parasitic infections and their association with socio-demographic status.
Materials and Methods
This was a cross-sectional study which was carried out at tertiary care hospital located in Central East India. Total 120 stool samples were collected from the immunosuppressive patients and were processed using direct wet mount preparation with saline and Lugol, formalin-ether concentration and Modified Ziehl-Neelsen stain. Different socio-demographic parameters were recorded. Statistical analysis was done using Graph Pad Prism version 8 Chi-square test. The p-value ≤0.05 was considered as statistically significant
Results
Out of total 120 patients, 20 (16.7%) were found to be infected. Majority of the population were males (66.7%). Among the total positive samples, 75% (15) showed the presence of Protozoa in which 10 samples had Entamoeba histolytica. The presence of Helminths was found in 25% (5), in which three samples showed Ascaris lumbricoides and two had Taenia species (10%).
Conclusion
The prevalence of parasitic infection among immunosuppressive patients in the present study was 16.7%. Entamoeba histolytica was the most commonly observed parasite. There was no significant association between prevalence of parasitic infections and socio-demographic data variables.
Amoebiasis, Formalin ether concentration technique, Helminth, Modified ziehl-neelsen stain
Introduction
Intestinal parasitic infection is observed as a major public health problem, especially in developing countries. With the increasing population of immunosuppressive people, it has become one of the major cause for morbidity and mortality is intestinal parasitic infections. It is observed as a major public health problem especially in developing countries. Parasitic infections like intestinal amoebiasis, giardiasis, ascariasis, tanieasis, crytosporidiasis and others result into diarrhea, gastro-intestinal and other systemic manifestations or the affected people may remain asymptomatic and may have carrier state. Socio-cultural milieu plays a significant role in these infections leading to adverse health effects [1]. In developing countries like India, socio-economic and environmental factors such as poor personal hygiene, poverty, lack of pure drinking water, malnutrition, poor health, sanitation, climate and overcrowding have been associated with parasitic infection [2,3]. Research shows that immune suppression, primary immunodeficiency and use of immunosuppressive drugs such as post-transplantation increases the risk of parasitic infection as chronic carriage stage [4]. However, a weak immune system can make a patient more prone to infectious diseases and parasitic infections [5]. Environmental changes, lack of clean water, diversity and density of population, lack of health hygiene close contact with infected animals is considered to be the most significant factors in the incidence and spread of parasite disease [6]. Globally, parasitic infections are common amongst the intestinal infections affecting annually about 3.5 billion people [7]. Moreover, parasitic infection may lead to poor immunity in infants, nutritional depletion, and loss of mucous membranes, lymphatic leakage and local hemorrhage [8]. Intestinal Parasitic Infections (IPI) are one of the public health problems in developing countries because of overcrowding, poor sanitation and low socio-economic status. Immunosuppressive individuals of poor and deprived communities of tropical and subtropical may have increased host susceptibility due to their pre-existing co-morbidities like HIV/AIDS, tuberculosis, diabetes and malnutrition. Health awareness, better personal hygiene and improved sanitation can offer prevention [9,10].
However, severity of the infection depends upon the species of parasite, type and course of infection, nature of interactions between the parasite and concurrent infections, nutritional and immunological status of host and different socio-economic factors [11]. According to World Health Organisation (WHO) amoebiasis caused by Entamoeba histolytica, is the most common parasitic infection in immunosuppressive patients with substantial morbidity and mortality followed by giardia caused by giardia spp and cryptosporidiosis caused by Cryptosporidium spp [12]. Ascaris lumbricoides, hookworm and Hymenolepis nana are the most common nematode and cestode causing infection [13].
Considering the importance of effective diagnosis and treatment of parasitic infected patients, direct microscopy for the detection of intestinal parasite disease is considered as useful and beneficial tool [14]. However, in immunosuppressed patients, low level of parasitemia can cause symptomatic consequences and severe complications, proper and specific laboratory diagnosis by serological and molecular based assays are important.
The aim of this study was to find out prevalence as well as association between parasite infections and their socio-demographic status in adult age group (>18 years) of immunocompromised individuals attending tertiary care hospital of Durg district, Chhattisgarh state.
Materials and Methods
The cross-sectional study was carried out in Department of Microbiology at a tertiary care hospital. The research study was approved by Sumandeep Vidyapeeth Institutional Ethics Committee. [SVIEC/ON/PhD/17018]. Patients on immunosuppressant; fulfilling inclusion criteria, during three months between June 2019 to September 2019 were studied. Patients were informed and appropriate consent was obtained before being recruited for the study.
Those patients who visited the hospital with illness like HIV, diabetes mellitus, tuberculosis, liver cirrhosis as well as positive Hepatitis B, taking steroid, on cytotoxic chemotherapy and immunosuppressant drugs etc., were considered as immunosuppressive patients and were included in the study. Inappropriate quality of stool sample was the exclusion criteria.
Total 120 immunosuppressive patients were included in this study. Each stool sample was collected in a clean, dry, leak proof, well-labeled wide mouth stool container with name and identification number and processed in the Parasitology section of the Microbiology department. Necessary data of patients were obtained from the Medical Record Department (MRD).
Macroscopic examination: Macroscopic analysis was done in terms of color and consistency and presence of adult worms as well as segments of tapeworm and larvae.
Microscopic examination: After macroscopic examination all samples were examined by direct microscopic method. Wet mount of the samples were performed as saline and iodine mounts to identify trophozoite and cysts of protozoan parasites as well as ova and larva. For the detection of intestinal coccidians Cryptosporidium parvum, Cyclospora and Isospora belli Modified Ziehl-Neelsen (MZN) stain was performed. Formal ether concentration technique was also done to detect eggs and larva of intestinal parasites [15].
Statistical Analysis
For the statistical analysis, Graph Pad Prism version 8 was used. Chi-square (χ2) test was performed and a p-value ≤0.05 was considered as statistically significant.
Results
Out of total 120 patients, 20 (16.7%) were found to be infected with parasite. Among all 120 immunosuppressive patients, 80 (66.7%) were males and 40 (33.3%) were females. Among the male population, 11 (9.2%) and among females, 9 (7.5%) were found to be positive for the infection. The age range of the population was 21-60 years, the mean age being 43.1±6.5 years. The age group of 41-50 years showed maximum parasitic infections. When two groups having highest (7 out of 46; 5.8% in age group of 41-50) and lowest prevalence (3 out of 27, 2.5% in age group 31-40) of parasitic infestation were compared; there was no statistical significant difference (p-value=0.73). No statistical significant difference was observed for socio-demographic variables like gender, age, education, employment, hygiene methods, (washing of hands before taking meals and after defecation), as well as with level of sanitation in form of defecation in open or having facility of toilet [Table/Fig-1].
Showing socio-demographic variables in frequency and percentage of intestinal parasites.
| Particulars | Frequency N (%) | Number of positive cases (%) | p-value |
|---|
| Gender |
| Male | 80 (66.7) | 11 (9.2) | 0.2253 |
| Female | 40 (33.3) | 9 (7.5) |
| Age (in Years) |
| 21-30 | 16 (13.3) | 6 (5.0) | 0.1123 |
| 31-40 | 27 (22.5) | 3 (2.5) |
| 41-50 | 46 (38.4) | 7 (5.8) |
| 51-60 | 31 (25.8) | 4 (3.3) |
| Education |
| Illiterate | 19 (15.8) | 5 (4.2) | 0.5765 |
| Primary school | 25 (20.8) | 4 (3.3) |
| High School | 32 (26.7) | 6 (5.0) |
| Diploma | 18 (15.0) | 3 (2.5) |
| Graduate | 15 (12.5) | 2 (1.7) |
| Post graduate | 11 (9.2) | 0 (0) |
| Job |
| Government | 15 (12.5) | 3 (2.5) | 0.6520 |
| Farmer | 46 (38.3) | 7 (5.8) |
| Student | 27 (22.5) | 3 (2.5) |
| Housewife | 23 (19.2) | 6 (5.0) |
| Other | 9 (7.5) | 1 (0.8) |
| Habit of washing hand |
| Before meal |
| Yes | 110 (91.7) | 17 (14.2) | 0.2373 |
| No | 10 (8.3) | 3 (2.5) |
| After defecation |
| Yes | 90 (75.0) | 11 (9.2) | 0.0237 |
| No | 30 (25.0) | 9 (7.5) |
| Use of toilet |
| Yes | 109 (90.8) | 16 (13.3) | 0.0659 |
| No | 11 (9.2) | 4 (3.3) |
Prevalence of parasitic infection was found to be high in HIV (24%), diabetes (14.75%), tuberculosis (17.65%) and patients having Hepatitis B infection (18.18%) while no parasitic infection was present in miscellaneous group [Table/Fig-2].
Details regarding the immunocompromised states of the study population.
| Immunocompromised status | Total number | Positive cases | Percentage (%) |
|---|
| HIV infection | 25 | 6 | 24.00 |
| Diabetes mellitus | 61 | 9 | 14.75 |
| Tuberculosis | 17 | 3 | 17.65 |
| Positive Hepatitis B | 11 | 2 | 18.18 |
| Miscellaneous group (Cirrhosis of liver (3), Chronic steroid therapy* (1), Cytotoxic chemotherapy with malignancy† (1), Immunosuppressant drugs‡ (1) | 6 (3+1+1+1) | -- | -- |
| Total | 120 | 20 | 16.67 |
*Prednisolone therapy for more than three months, †Imatinib therapy for CML for last one year. ‡Azathioprine (AZA) for SLE,
As shown in [Table/Fig-3], among the total 20 positive samples; 15 samples (75%) showed the presence of Protozoa in which 10 samples (50%) showed presence of Entamoeba histolytica [Table/Fig-4], followed by Cryptosporidium parvum [Table/Fig-5] in four samples (20%) and Giardia lamblia in one sample (5%). The presence of Helminths was found in five samples (25%), of which three samples (15%) showed the presence of Ascaris lumbricoides [Table/Fig-6] and Taenia species eggs [Table/Fig-7] were found in two samples (10%) [Table/Fig-3]. Prevalence ratio of protozoan to helminths parasites was 3:1.
Showing total number of parasite identified.
| Parasite | Number of samples with parasites | Percentage (%) |
|---|
| Protozoa |
| Entamoeba histolytica | 10 | 50.0 |
| Giardia lamblia | 1 | 5.0 |
| Cryptosporidium parvum | 4 | 20.0 |
| Helminths |
| Ascaris lumbricoides | 3 | 15.0 |
| Taenia species | 2 | 10.0 |
| Total | 20 | 16.7 (20/120) |
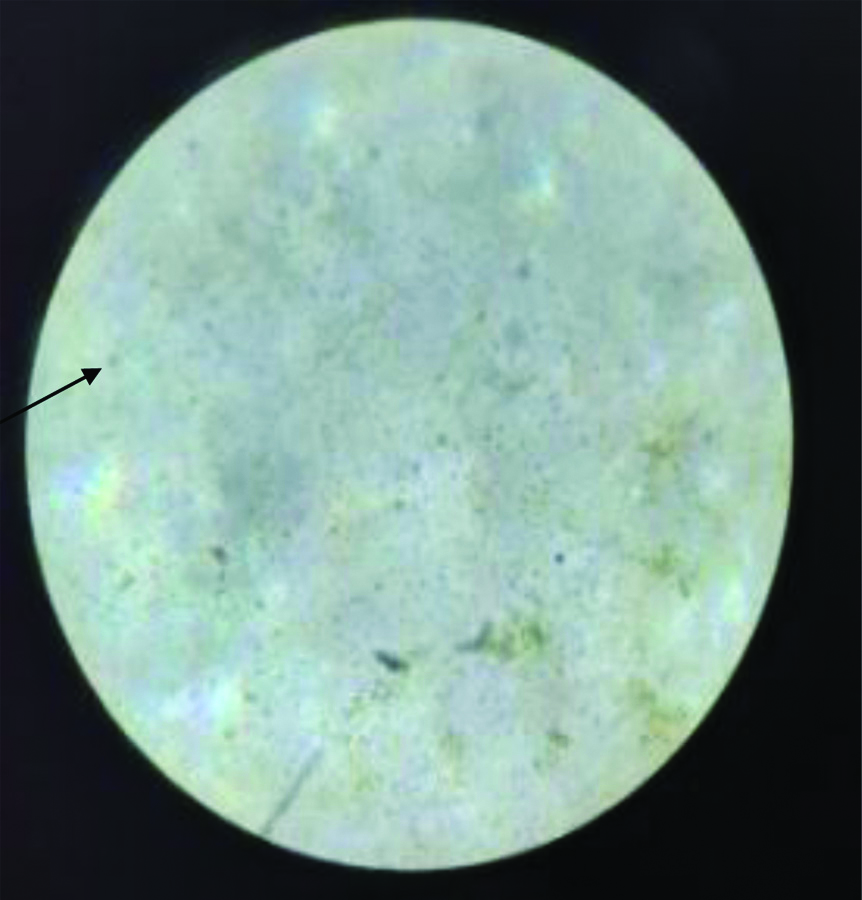
Stool microscopy showing cyst of Entamoeba histolytica in saline wet mount (40X).
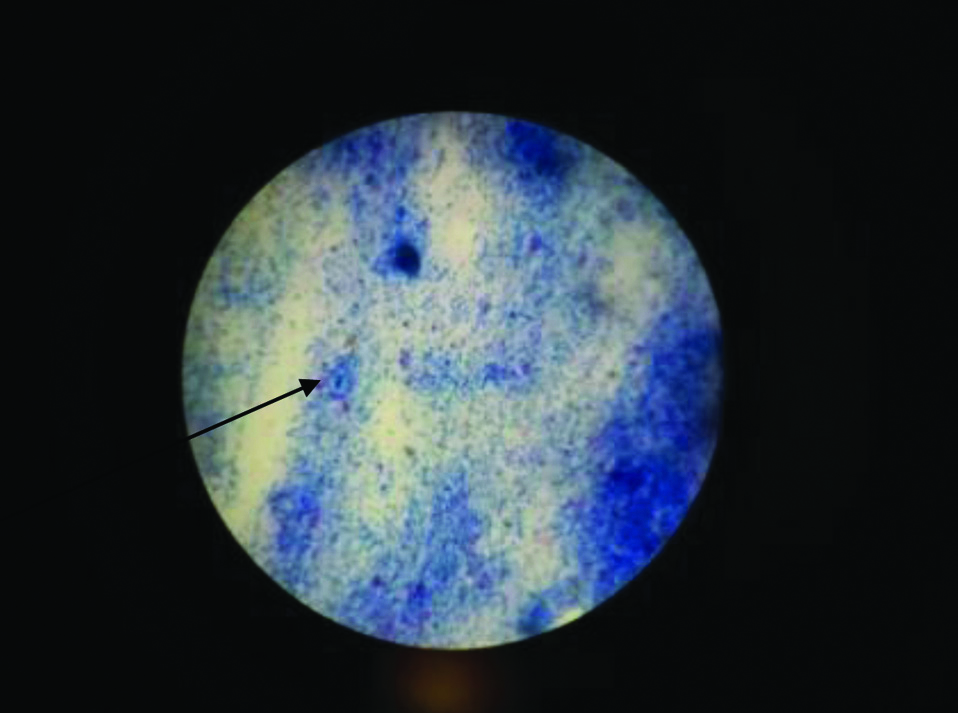
Modified ZN stain showing Cryptosporidium parvum in stool sample under oil immersion field (100X power).
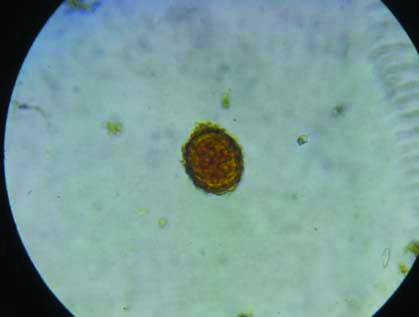
Stool microscopy showing ova of Ascaris lumbricoides in saline wet mount (40X).
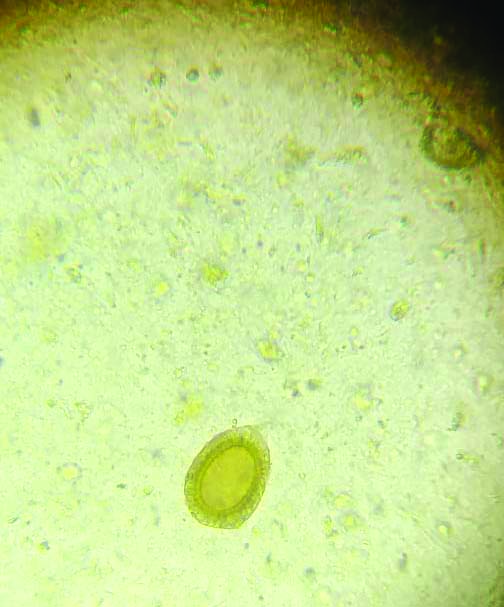
Stool microscopy showing Taenia species ovum in saline wet mount (40X).
Discussion
In this study the overall prevalence of parasitic infection in immunosuppressive patients was 16.7%. This study was primarily done to find out the risk factors involved in developing parasitic infection in immunosuppressive patients. Study done by Hailegebriel T showed that various socio-demographic factors like size of the family, hygienic practices like hand washing, nail cutting and wearing shoes had relation with intestinal parasitic infection [16]. In immunosuppressed patients, if same holds true, then there will be the need for additional interventional strategy. Moudgil V et al., in their study found co-prevalence of diabetes with parasitic infection of 8.6% [17]. In a study by Sabah AA and Temsah AG they showed parasitic infection amongst diabetic patients had predominance of E. histolytica, however their was no significant difference between patients having diabetes and controls [18]. They assumed that E. histolytica can be a factor for poor sanitation and environment pollution. Predominance of E. histolytica, was found in present study also however no significant relation was found with socio-demographic risk factors. It can therefore be presumed that immunosuppression was responsible for parasitic infections than socio-demographic factors; however a larger population based study may be needed for significant conclusion. Immunosuppression due to HIV may also have opportunistic infection by enteric parasite. In this study of 120 patients having immunosuppression, 24 were HIV positive of which 6 had parasitic infection (25%). Swathirajan CR et al., studied 829 HIV positive individuals of which 23.4% had enteric parasitic infections [19]. Study done by Ghoshal U et al., on detection of opportunistic parasitic pathogens in immunocompromised patients, of 10,233 stool samples received in 11 years by Institute parasitology laboratory, 64 were found to excrete oocysts of Cystoisospora, of which 37/64 (57.81%) were HIV positive [20]. The prevalence of parasitic infection varies between different countries, places or even regions. Hence, present study shows relatively lower prevalence than other studies. This could be because of different method adopted for finding out this data.
In this study, Entamoeba histolytica was the most common parasite observed followed by Cryptosporidium parvum, Ascaris lumbricoides, Taenia species and Giardia lamblia was least observed parasite. Study carried out in immunocompetent and immunocopromised patients have different parasitic infestations; like opportunistic enteric parasitic infections like cystoisosporiasis is greater in HIV positive than in negative individuals [19]. In another study by Kaniyarakkal V et al., [21] on 200 seropositive HIV patients, Cystoisospora and Cryptosporidium spp were most commonly observed parasite followed by Microsporidium spores and Chilomastix mesnili. Cystoisospora spp, common opportunistic parasitic infestation in HIV positive individuals can cause diarrhoea and may be related to poor sanitation and lack of hygiene. Cryptosporidium and Microsporidium, are associated with severe immunosuppression that may require high index of suspicion and need different staining methods for diagnosis.
Study carried out by Saraswathi R et al., from patients of different speciality units of rural teaching hospital, 55 of 726 stool samples (7.6%) were positive for intestinal parasites [22]. In present study, of 120 stool samples of immunocompromised patients, 20 (16.7%) had parasitic infection. Present study and other studies have reported higher prevalence of intestinal parasites in immunocompromised patients than reported by studies in immunocompetent and general population [18,19,22,23]. Gender related distribution of parasitic infection is reported higher in males in comparison with females in general population, for the reason that males are more exposed to environment [14,22]. There is lack of specific gender related intestinal parasite prevalence data and also of their relation to socio-demograhic risk factors in immunocopromised individuals. Present study did not show significant gender wise difference and it also did not show any significant connection between the prevalence of parasite infections and socio-demographic variables of immunocopromised patients. Study by Shahdoust S et al., showed no significant association between the prevalence of parasitic infestation and the variables of socio-demographic data in patients who were referred to hospital of Tonekabon Mazandaran province, Iran [14]. Studies done in general population as well as in immunocompromised patients recommend for improvement on local risk factors like poor sanitation, contact with soil, contact with domestic animal and others, which requires community education in health and hygiene [5,23,24].
In present study of 120 immunosuppressed patients, 61 had DM of which 9 (14.75%) had intestinal parasitic infection. In a case control study by Mohtashamipour M et al., on 118 DM patients and same number of control group healthy individuals, parasitic infection prevalence was 26.3% in diabetic patients which was significantly higher than in control group [5].
Immunosuppression is an important risk factor for development of parasitic infections. Though some of the evidences generated in general population can be broadly applied to immunosuppressed patients, there seems to be a distinctive and separate pathophysiological mechanism, prevalence, profile and type of parasite involvement in immunosuppressed patients [16,17,19,20]. For better management of such patients, diseases causing immunosuppression and parasitic involvement, like diabetes should be linked with blood sugar and HbA1c control; HIV/AIDS to be related to CD4 count and ART treatment and post transplant as well as patients having malignancy to type and duration of immunomodulators/chemotherapeutic/drugs [4,5,10,11,18].
Limitation(s)
One of the limitations of the study was a small sample size. Larger sample with more representation of different groups of immunosuppressed patients like Diabetes/HIV/AIDS/Post transplant group can give better insight.
Conclusion(s)
The prevalence of parasite infection among immunosuppressive patients was 16.7% in this study. Entamoeba histolytica was the most common observed parasite followed by Cryptosporidium parvum. There was no significant relation between socio-demographic risk factor and parasitic infections in immunosuppressed patients. Mostly of the time parasitic infection can be diagnosed by stool microscopy. Risk factors and profile of parasitic infections can vary in immunosuppressed patients, which require further research so that treatment and prevention can help to decrease the morbidity in immunosuppressed patients.
*Prednisolone therapy for more than three months, †Imatinib therapy for CML for last one year. ‡Azathioprine (AZA) for SLE,
Author Declaration:
Financial or Other Competing Interests: None
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Oct 27, 2020
Manual Googling: Nov 21, 2020
iThenticate Software: Dec 22, 2020 (11%)
[1]. Hicks JH, Kremer M, Miguel E, The case for mass treatment of intestinal helminths in endemic areasPLoS Negl Trop Dis 2015 22:e000421410.1371/journal.pntd.000421426492528 [Google Scholar] [CrossRef] [PubMed]
[2]. Chandi DH, Lakhani SJ, Prevalence of parasitic infections among school children in Bhaili, Durg Chhattisgarh, IndiaInt J Curr Microbiol App Sci 2018 7(9):1919-25.10.20546/ijcmas.2018.709.233 [Google Scholar] [CrossRef]
[3]. Nyundo AA, Munisi DZ, Gesase AP, Prevalence and correlates of intestinal parasites among patients admitted to Mirembe National Mental Health Hospital, Dodoma, TanzaniaJ Parasitol Res 2017 2017:565171710.1155/2017/565171728611925 [Google Scholar] [CrossRef] [PubMed]
[4]. Aulagnon F, Scemla A, DeWolf S, Legendre C, Zuber J, Diarrhea after kidney transplantation: A new look at a frequent symptomTransplantation 2014 98(8):806-16.10.1097/TP.000000000000033525073040 [Google Scholar] [CrossRef] [PubMed]
[5]. Mohtashamipour M, Hoseini SG, Pestehchian N, Yousefi H, Fallah E, Hazratian T, Intestinal parasitic infections in patients with diabetes mellitus: A case-control studyJ Res in Clin Med 2015 3(3):157-63.10.15171/jarcm.2015.025 [Google Scholar] [CrossRef]
[6]. Belhassen-García M, Pardo-Lledías J, Pérez Del Villar L, Velasco-Tirado V, Siller Ruiz M, Cordero-Sánchez M, Screening for parasite infections in immigrant children from low-income countriesEnferm Infecc Microbiol Clin 2017 35(1):27-32.10.1016/j.eimc.2016.03.00727156246 [Google Scholar] [CrossRef] [PubMed]
[7]. Tigabu A, Taye S, Aynalem M, Adane K, Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura Health Center, Northwest EthiopiaBMC Research Notes 2019 12(1):33310.1186/s13104-019-4377-y31186041 [Google Scholar] [CrossRef] [PubMed]
[8]. Patel MM, Patel PR, Gamit B, Modi J, Padsala S, Prevalence of intestinal parasites infestation in Surat city of South Gujarat: A hospital based studyNatl J Community Med 2014 5(3):273-75. [Google Scholar]
[9]. Kuété T, Yemeli FL, Mvoa EE, Nkoa T, Somo R, Ekobo AS, Prevalence and risk factors of intestinal helminth and protozoa infections in an urban setting of Cameroon: The case of DoualaAm J Epidemiol Infect Dis 2015 3(2):36-44. [Google Scholar]
[10]. Gebrewahid T, Gebrekirstos G, Teweldemedhin M, Gebreyesus H, Awala A, Tadla K, Intestinal parasitosis in relation to CD4 count and anemia among ART initiated patients in St. Mary Aksum general hospital, Tigray, EthiopiaBMC Infect Dis 2019 19(1):35010.1186/s12879-019-3989-031029088 [Google Scholar] [CrossRef] [PubMed]
[11]. Srirangaraj S, Venkatesha D, Opportunistic infections in relation to antiretroviral status among AIDS patients from south IndiaIndian J Med Microbiol 2011 29(4):39510.4103/0255-0857.90175 [Google Scholar] [CrossRef]
[12]. World Health OrganizationPrevention and Control of Intestinal Parasitic Infections 2002 GenevaWHO [Google Scholar]
[13]. Langbang D, Dhodapkar R, Parija SC, Premarajan KC, Rajkumari N, Prevalence of intestinal parasites among rural and urban population in Puducherry, South India-a community-based studyJ Family Med Prim Care 2019 8(5):160710.4103/jfmpc.jfmpc_196_1931198723 [Google Scholar] [CrossRef] [PubMed]
[14]. Shahdoust S, Niyyati M, Haghighi A, Azargashb E, Khataminejad MR, Prevalence of intestinal parasites in referred individuals to the medical centers of Tonekabon city, Mazandaran provinceGastroenterol Hepatol Bed Bench 2016 9(Suppl1):S75 [Google Scholar]
[15]. Tille P, Bailey & Scott’s diagnostic microbiology 2015 Dec 28 13th editionElsevier Health Sciences [Google Scholar]
[16]. Hailegebriel T, Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, EthiopiaBMC Infect Dis 2017 17(1):36210.1186/s12879-017-2466-x28535750 [Google Scholar] [CrossRef] [PubMed]
[17]. Moudgil V, Rana R, Tripathi PK, Farooq U, Sehgal R, Khan MA, Coprevalence of parasitic infections and diabetes in Sub-Himalayan region of Northern IndiaInt J Health Sci (Qassim) 2019 13(1):19 [Google Scholar]
[18]. Sabah AA, Temsah AG, Prevalence of some gastro-intestinal parasites in diabetic patients in tanta city, Gharbia Governorate, EgyptJ Egypt Soc Parasitol 2015 240(2496):1-4. [Google Scholar]
[19]. Swathirajan CR, Vignesh R, Pradeep A, Solomon SS, Solomon S, Balakrishnan P, Occurrence of enteric parasitic infections among HIV-infected individuals and its relation to CD4 T-cell counts with a special emphasis on coccidian parasites at a tertiary care centre in South IndiaIndian J Med Microbiol 2017 35(1):3710.4103/ijmm.IJMM_16_16428303816 [Google Scholar] [CrossRef] [PubMed]
[20]. Ghoshal U, Jain V, Tejan N, Kalra SK, Ranjan P, Sinha R, A road less travelled: Clinical comparison of HIV seropositive and seronegative patients with cystoisosporiasis–An 11-year experience from a tertiary care centre in Northern IndiaIndian J Med Microbiol 2018 36(4):50810.4103/ijmm.IJMM_18_9930880697 [Google Scholar] [CrossRef] [PubMed]
[21]. Kaniyarakkal V, Mundangalam N, Moorkoth AP, Mathew S, Intestinal parasite profile in the stool of HIV positive patients in relation to immune status and comparison of various diagnostic techniques with special reference to Cryptosporidium at a tertiary care hospital in South IndiaAdvances in Medicine 2016 201610.1155/2016/356435927493988 [Google Scholar] [CrossRef] [PubMed]
[22]. Saraswathi R, Lalithambigai J, Vazhavandal G, Uma A, Prabhusaran N, Velayutharaj A, Prevalence of intestinal parasitic pathogens in stools from medical speciality units of tertiary rural teaching hospital, Tamilnadu, IndiaInt J Curr Microbiol App Sci 2016 5:429-35.10.20546/ijcmas.2016.508.046 [Google Scholar] [CrossRef]
[23]. Kiani H, Haghighi A, Rostami A, Azargashb E, Tabaei SJ, Solgi A, Zebardast N, Prevalence, risk factors and symptoms associated to intestinal parasite infections among patients with gastrointestinal disorders in Nahavand, Western IranRev Inst Med Trop Sao Paulo 2016 58:4210.1590/S1678-994620165804227253744 [Google Scholar] [CrossRef] [PubMed]
[24]. Lander RL, Lander AG, Houghton L, Williams SM, Costa-Ribeiro H, Barreto DL, Mattos AP, Gibson RS, Factors influencing growth and intestinal parasitic infections in preschoolers attending philanthropic daycare centers in Salvador, Northeast Region of BrazilCad Saude Publica 2012 28:2177-88.10.1590/S0102-311X201200110001723147959 [Google Scholar] [CrossRef] [PubMed]