Chemotherapy Induced Peripheral Neuropathy (CIPN) is a serious adverse effect of drugs like paclitaxel, vincristine, oxaliplatin and bortezomib [1]. The debilitating and painful symptoms like spontaneous pain, allodynia and hyperalgesia result in noncompliance of chemotherapy schedule by cancer patients. The management of CIPN is mostly unsatisfactory. A variety of drugs like anticonvulsants, antidepressants, opioids, local anaesthetics and antioxidants are employed to treat CIPN [2] however, with only partial success. Moreover, the above drugs also produce various adverse effects confounding the treatment paradigm.
Thus, there is an urgent requirement for newer agents to overcome CIPN. Such new drugs per se should be free from serious adverse effects to ensure proper adherence to chemotherapeutic schedule. Though the aetiopathogenesis of CIPN is complex, extensive preclinical research provides evidences for three main neuronal toxic effects of cancer chemotherapeutic drugs. These drugs may target mitochondria and cause severe oxidative stress, produce functional impairment of ion channels and trigger inflammatory mechanisms through the activation of glial cells [3]. Flavone compounds are naturally occurring antioxidants [4] and the anti-inflammatory activity of many flavone derivatives has been documented [5,6]. Considering these beneficial effects, a few flavonoids like myricetin, quercetin and rutin have been investigated and found to be effective in animal models of neuropathy [7,8]. Recently, 6-methoxyflavone has been reported to suppress the manifestations of cisplatin induced peripheral neuropathy in rodents [9].
Flavone and its monohydroxy derivatives have been found to possess significant antinociceptive effect in mice [10] and potent anti-inflammatory effect in rats [5]. However, these compounds have not been investigated for their effect on CIPN. Hence, the main aim of the present study was to investigate the effect of flavone and a few structurally related monohydroxy flavones (5-hydroxy flavone, 6-hydroxy flavone and 7-hydroxy flavone) for their potential effect in CIPN induced by two most commonly used chemotherapeutic drugs vincristine and oxaliplatin in mice.
Materials and Methods
Animals
This experimental animal study was carried out in the Department of Pharmacology, Meenakshi Medical College Hospital and Research Institute, Kanchipuram between April 2015 and March 2017.
Adult male Swiss albino mice (20-25 g) were used in the present study. The animals were maintained in a controlled environment (24±1°C) with 12 hrs/12 hrs day/night cycle starting at 7 am with free access to food and water. All the experiments were carried out between 09.00 and 13.00 hour to avoid circadian variation. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC No-KN/COL/3408/2014 dt. 22.07.2014). The guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA, India) were adhered to in performing the experiments.
Drugs and Chemicals
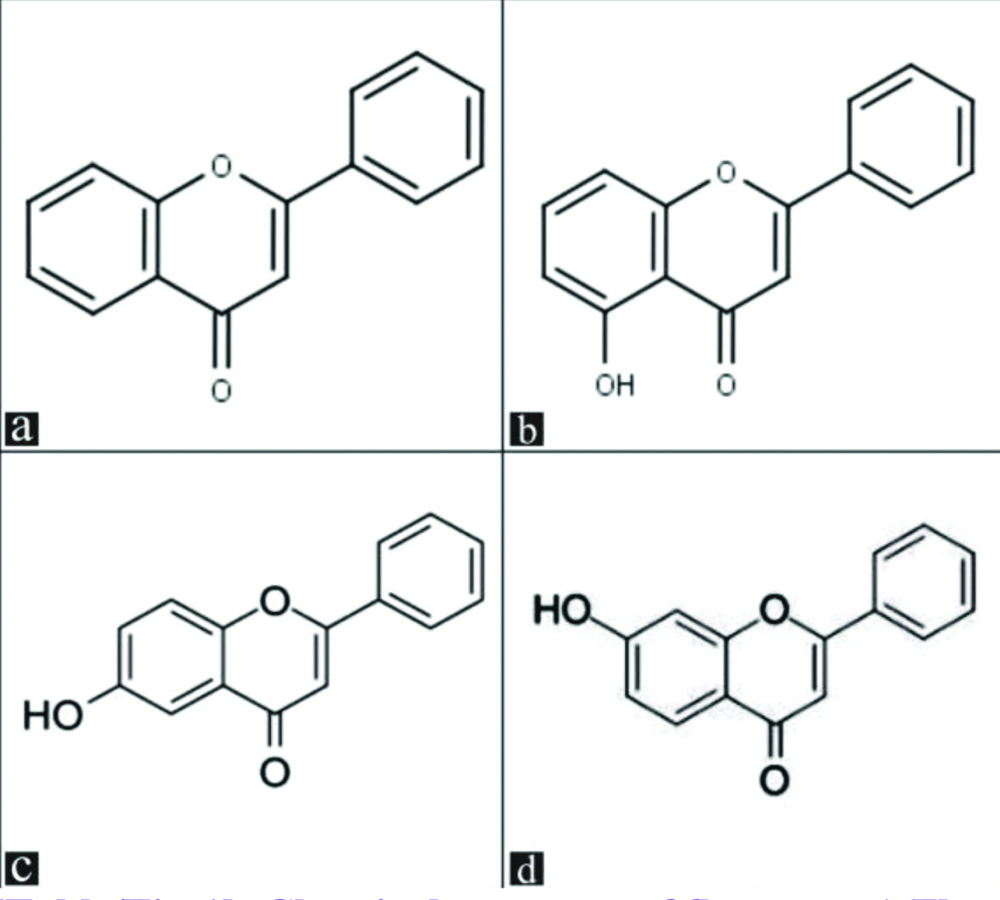
Flavone, 5-hydroxy flavone, 6-hydroxy flavone and 7-hydroxy flavone [Table/Fig-1] were procured from Research Organics, Chennai. Being water insoluble, the above flavone derivatives were prepared as a fine suspension in 0.5% Carboxy Methylcellulose (CMC) for subcutaneous (s.c) injection in mice. Vincristine (vincristine sulfate USP, Cipla, India) and oxaliplatin (Oxaliplatin USP, Sun Pharma, India) were used to induce CIPN. Acetone (Merck specialities Pvt., Ltd., India) was used for the assessment of cold allodynia. Morphine (Morphine sulphate, PharmaChemico laboratories, India) was used as a standard analgesic. Vincristine, oxaliplatin and morphine were diluted with distilled water.
Chemical structure of flavones: a) Flavone; b) 5-hydroxy flavone; c) 6-hydroxy flavone; d) 7-hydroxy flavone.
(Source: Research Organics, Chennai)
Experimental Design-Induction of CIPN and drug treatment
Group I (n=6)
Vincristine (0.1 mg/kg I.P)
Vehicle- 0.5% carboxymethyl cellulose s.c
Morphine- 10 mg/kg, s.c
Flavone (50,100,200 mg/kg) s.c
5-hydroxy flavone (50,100,200 mg/kg) s.c
6-hydroxy flavone (50,100,200 mg/kg) s.c
7-hydroxy flavone (50,100,200 mg/kg) s.c
Group II
Oxaliplatin (6 mg/kg I.P)
Vehicle- 0.5% carboxymethyl cellulose s.c
Morphine- 10 mg/kg, s.c
Flavone (50,100,200 mg/kg) s.c
5-hydroxy flavone (50,100,200 mg/kg) s.c
6-hydroxy flavone (50,100,200 mg/kg) s.c
7-hydroxy flavone (50,100,200 mg/kg) s.c
The CIPN was induced in separate groups of randomly selected mice with different treatment schedules of oxaliplatin and vincristine. Oxaliplatin in a dose of 6 mg/kg was injected intraperitoneally (I.P) in mice and neuropathic manifestations, viz., mechanical allodynia, cold allodynia and thermal hyperalgesia were assessed 72 hour later [11]. Vincristine 0.1 mg/kg was administered I.P to mice daily for seven days and the above mentioned behavioural responses were assessed on the 8th day [12]. After induction of CIPN in mice with either oxaliplatin or vincristine, they were randomly allocated to different treatment groups by using table of random numbers. {Flavone, 5-hydroxy flavone, 6-hydroxy flavone, 7-hydroxy flavone, vehicle (0.5% CMC, s.c) and morphine (10 mg/kg s.c)}. The doses of the flavone compounds (50,100 and 200 mg/kg s.c) were selected based on the antinociceptive study reported earlier [10]. Each group consisted of six mice. The behavioural responses like mechanical allodynia, cold allodynia and thermal hyperalgesia in the above groups of mice were evaluated prior to and 30 minutes after respective treatments. The behavioural tests were assessed by an experienced experimenter who was blinded to the treatments provided in either group.
Assessment of Mechanical Allodynia [
13]
Mice were placed individually in a plastic cage (13x7x7 cm) with a wire mesh bottom. After an acclimatisation period of 15 minutes, a fine von Frey’s filament with a bending force of 0.69 mN was applied perpendicularly against the plantar surface of the hind paws of mice and was held for 1-3 second. The response to the stimulus was ranked as follows:
0-No response
1-Move away from the filament
2-Immediate flinching or licking of the hind paw
Stimulation with von Frey’s hair aesthesiometer was applied five times to each hind paw at an interval of 30 seccond and the sum of ten values was considered as paw withdrawal response score.
Assessment of Cold Allodynia [
14]
Mice were placed individually in a plastic cage with a wire mesh bottom and acclimatised for a period of 15 minutes. Cold allodynia was assessed by touching each hind paw of the mouse with a bubble of acetone formed at the tip of a 1 mL syringe. The response of the mouse to acetone was noted for a period of 20 second and was graded on a four-point scale:
0-No response
1-Immediate withdrawal
2-Prolonged withdrawal
3-Licking/biting of the paw
Acetone bubble was applied thrice to the hind paw with a gap of 1 min between each application and the individual scores from both hind paws were added to obtain a paw withdrawal response score.
Thermal Hyperalgesia [
15]
Hot water tail immersion test was conducted for assessment of thermal hyperalgesia. The mouse was restrained in a mouse holder and the tail was immersed upto1cm from the base in a hot water bath maintained at 48±0.5°C. The time taken for the mouse to flick the tail from hot water was considered as the reaction time. The reaction time was noted initially before any drug treatment and 30 minutes after drug treatment. A significant increase in mean reaction time between these two readings is an indication of attenuation of thermal hyperalgesia. A cut-off time of 20 seconds was maintained to prevent any injury to the tail.
Statistical Analysis
The results were analysed by one-way Analysis of Variance (ANOVA) followed by Dunnett’s t-test for multiple comparison or paired t-test using Statistical Package for the Social Sciences (SPSS) version16. A p-value less than 0.05 was considered statistically significant.
Results
Effect of flavone and monohydroxy derivatives on peripheral neuropathy induced by vincristine and oxaliplatin.
Administration of either vincristine or oxaliplatin in mice resulted in the manifestation of characteristic symptoms of peripheral neuropathy like mechanical allodynia, cold allodynia and thermal hyperalgesia. The above responses were differentially modified by treatment with morphine, flavone or its monohydroxy derivatives.
Mechanical Allodynia
Aversive behavioural responses like flinching or lifting of hind paws were recorded when von Frey’s hair aesthesiometer was applied to vincristine or oxaliplatin administered mice.
In vincristine administered mice, the mean paw withdrawal response score after vehicle treatment was not significantly different compared to its pretreatment value (p>0.05) [Table/Fig-2].
Effect of flavone and monohydroxy flavones on vincristine-induced mechanical allodynia in mice.
| Group-I n=6 | Paw withdrawal response scores after various treatments (mean±SEM) |
|---|
| Dose mg/kgs.c | Flavone | 5-hydroxy flavone | 6-hydroxy flavone | 7-hydroxy flavone |
|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment |
|---|
| 50 | 18.66±0.91 | 18.50±1.31 | 19.00±0.68 | 18.83±0.65 | 18.50±0.61 | 19.00±0.44 | 19.33±0.49 | 19.66±0.33 |
| 100 | 19.83±0.16 | 17.00±1.09 | 20.00±0.00 | 17.00±1.43 | 19.16±0.65 | 15.66±0.88* | 19.83±0.16 | 17.16±0.90* |
| 200 | 19.66±0.33 | 8.00±1.03* | 19.37±0.49 | 14.00±2.69* | 20.00±0.00 | 15.33±1.99* | 19.33±0.42 | 14.50±1.02* |
* p<0.05 compared to the corresponding value before drug treatment (Paired t-test)
The paw withdrawal response scores in vehicle treated mice were 19.16±0.40 and 19.33±0.33 before and after treatment
The paw withdrawal response scores in morphine (10 mg/kg, s.c) treated mice were 18.66±0.66 and 7.33±0.84*before and after treatment
Treatment with morphine resulted in a significant reduction in the paw withdrawal response score (18.66±0.66 and 7.33±0.84). Treatment with flavone or its monohydroxy derivatives resulted in a decrease in the mean paw withdrawal response score compared to their pretreatment values. The reduction in the score was statistically significant only in a dose of 200 mg/kg of flavone, 5-hydroxy flavone and 7-hydroxy flavone (p<0.05) [Table/Fig-2]. A significant reduction of the score was evident with 100 mg/kg of 6-hydroxy flavone, however, there was no further reduction with increase in dose.
In animals that received oxaliplatin, a maximum paw withdrawal response score (20.00±0.00) was recorded that was not significantly altered by vehicle treatment [Table/Fig-3]. A marked decrease in mean paw withdrawal response score was produced by morphine treatment in these animals (18.16±0.90 and 2.66±1.76, p<0.05). A significant reduction of paw withdrawal response score was evident in mice treated with 5-hydroxy flavone in doses of 100 mg/kg and 200 mg/kg (p<0.05). A higher dose of 200 mg/kg of flavone, and 6-hydroxy flavone was required to produce a significant reduction (p<0.05). However, 7-hydroxy flavone treatment did not result in any significant change in paw withdrawal response score in the doses employed.
Effect of flavone and monohydroxy flavones on oxaliplatin-induced mechanical allodynia in mice.
| Group II n=6 | Paw withdrawal response scores after various treatments (mean±SEM) |
|---|
| Dose mg/kg, s.c | Flavone | 5-hydroxy flavone | 6-hydroxy flavone | 7-hydroxy flavone |
|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment |
|---|
| 50 | 18.33±0.74 | 17.16±1.27 | 19.83±0.16 | 19.50±0.50 | 19.16±0.40 | 19.16±0.54 | 18.83±0.65 | 19.16±0.65 |
| 100 | 20.00±0.00 | 17.16±1.32 | 19.33±0.66 | 15.16±1.75* | 20.00±0.00 | 18.16±0.83 | 19.66±0.33 | 20.00±0.00 |
| 200 | 19.33 ±0.33 | 12.67 ±1.30* | 18.83±0.54 | 13.50±1.28* | 18.50±0.61 | 14.00±1.52* | 19.66±0.33 | 18.66±0.98 |
* p<0.05 compared to the corresponding value before drug treatment (Paired t-test)
The paw withdrawal response scores in vehicle treated mice were 20.00±0.00 and 19.66±0.33 before and after treatment
The paw withdrawal response scores in morphine (10 mg/kg,s.c) treated mice were 18.16±0.90 and 2.66±1.76* before and after treatment
Cold Allodynia
Application of a drop of acetone on the hind paw of vincristine or oxaliplatin administered mice elicited an aversive response like immediate withdrawal of the paw with licking or biting behaviour.
In vincristine administered animals, the paw withdrawal response score was not altered by vehicle treatment. However, a significant reduction (p<0.05) in paw withdrawal response score was evident after morphine treatment when compared to its pretreatment value [Table/Fig-4].
Effect of flavone and monohydroxy flavones on vincristine-induced cold allodynia in mice.
| Group-I n=6 | Paw withdrawal response scores after various treatments (mean±SEM) |
|---|
| Dose mg/kg, s.c | Flavone | 5-hydroxy flavone | 6-hydroxy flavone | 7-hydroxy flavone |
|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment |
|---|
| 50 | 15.33±1.05 | 11.33±1.40* | 16.50±0.34 | 13.33±0.98* | 19.16±0.65 | 15.66±0.88* | 14.00±0.70 | 13.66±1.08 |
| 100 | 15.83±0.94 | 6.83±1.13* | 16.83±0.30 | 10.83±1.22* | 16.00±0.57 | 13.83±1.32* | 17.00±0.51 | 13.50±0.50* |
| 200 | 17.33±0.33 | 5.16±1.70* | 17.33±0.42 | 10.33±1.46* | 16.83±0.79 | 9.00±1.96* | 15.00±0.81 | 10.66±1.33* |
* p<0.05 compared to the corresponding value before drug treatment (Paired t-test)
The paw withdrawal response scores in vehicle treated mice were 16.33±0.40 and 15.66±0.55 before and after treatment
The paw withdrawal response scores in morphine (10 mg/kg, s.c) treated mice were 16.50±0.56 and 5.00±1.75*before and after treatment
Treatment with flavone, 5-hydroxy flavone and 6-hydroxy flavone significantly reduced the paw withdrawal response scores due to cold allodynia in all the doses studied (p<0.05). 7-hydroxy flavone offered a significant reduction in paw withdrawal response score only in doses of 100 and 200 mg/kg [Table/Fig-4]. Flavone treatment resulted in a greater reduction of paw withdrawal response score compared to its monohydroxy derivatives.
The paw withdrawal response score due to cold allodynia in oxaliplatin administered animals was greatly attenuated by morphine treatment [Table/Fig-5].
Effect of flavone and monohydroxy flavones on oxaliplatin-induced cold allodynia in mice.
| Group II n=6 | Paw withdrawal response scores after various treatments (mean±SEM) |
|---|
| Dose mg/kg, s.c | Flavone | 5-hydroxy flavone | 6-hydroxy flavone | 7-hydroxy flavone |
|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment |
|---|
| 50 | 16.33±0.33 | 13.50±0.95 | 16.16±0.47 | 15.33±0.42 | 17.00±0.51 | 15.33±0.74 | 14.16±1.85 | 13.16±1.88 |
| 100 | 16.50±0.34 | 10.33±1.70* | 15.50±1.02 | 15.66±0.88 | 16.66±0.84 | 14.33±0.84 | 15.83±0.60 | 11.16±1.04* |
| 200 | 15.67 ±0.42 | 9.17±0.70* | 15.16±1.01 | 8.83±1.01* | 15.33±0.98 | 10.33±1.25* | 16.83±0.30 | 11.33±1.54* |
* p<0.05 compared to the corresponding value before drug treatment (Paired t-test)
The paw withdrawal response scores in vehicle treated mice were 17.00±0.68 and 16.00±0.57 before and after treatment.
The paw withdrawal response scores in morphine (10 mg/kg, s.c) treated mice were 15.50±0.99 and 0.16±0.16* before and after treatment.
A significant reduction (p<0.05) in paw withdrawal response score was also observed after treatment with flavone and 7-hydroxy flavone in doses of 100 and 200 mg/kg. However, a statistically significant reduction of paw withdrawal response score was evident with 5-hydroxy flavone and 6-hydroxy flavone only in a dose of 200 mg/kg [Table/Fig-5].
Thermal Hyperalgesia
Vehicle treatment resulted in an increase of 0.81±0.67 seconds in the mean reaction time for tail withdrawal when thermal hyperalgesia was assessed in vincristine administered mice [Table/Fig-6]. Morphine treatment markedly increased the reaction time for tail withdrawal (16.93±1.07sec). A significant increase in reaction time was also recorded in animals treated with flavone in doses of 100 and 200 mg/kg. Treatment with 5-hydroxy flavone and 6-hydroxy flavone could offer a statistically significant increase in reaction time only in a dose of 200 mg/kg (p<0.05) [Table/Fig-6]. Though an increase in mean reaction time was observed in 7-hydroxy flavone treated mice, the values were not statistically significant (p>0.05).
Effect of flavone and monohydroxy flavones on vincristine-induced thermal hyperalgesia in mice.
| Group-I n=6 | Mean increase in reaction time (sec) for tail withdrawal (mean±SEM) |
|---|
| Dose mg/kg, s.c | Flavone | 5-hydroxy flavone | 6-hydroxy flavone | 7-hydroxy flavone |
|---|
| 50 | 1.13±0.29 | 2.60±0.87 | 0.63±0.21 | 1.07±0.96 |
| 100 | 4.79±0.87* | 2.55±0.64 | 1.84±0.19 | 1.69±0.21 |
| 200 | 6.22±1.43* | 3.02±0.87* | 3.77±0.32* | 1.99±0.33 |
* p<0.05 compared with vehicle treatment (ANOVA, Dunnett’s t-test)
Mean increase in reaction time for tail withdrawal in vehicle treated mice was 0.81±0.67sec and morphine (10 mg/kg, s.c) treated mice was 16.93±1.07*sec
In oxaliplatin treated mice, morphine treatment markedly increased the reaction time for tail withdrawal (16.80±0.94 sec, p<0.05) compared to vehicle treatment (0.43±0.61 sec) [Table/Fig-7]. A dose dependent increase in the reaction time for tail withdrawal was observed after treatment with monohydroxy flavone. The increase in reaction time was statistically significant (p<0.05) for flavone only in a dose of 200 mg/kg, whereas, 5-hydroxy flavone, 6-hydroxy flavone and 7-hydroxy flavone produced a significant increase in a dose of 100 mg/kg. 7-hydroxy flavone treatment offered a significant protection against thermal hyperalgesia in oxaliplatin treated mice but it was not observed in vincristine treated mice.
Effect of flavone and monohydroxy flavones on oxaliplatin-induced thermal hyperalgesia in mice.
| Group II n=6 | Mean increase in reaction time(sec) for tail withdrawal (mean±SEM) |
|---|
| Dose mg/kg, s.c | Flavone | 5-hydroxy flavone | 6-hydroxy flavone | 7-hydroxy flavone |
|---|
| 50 | 1.73±0.41 | 2.95±0.33 | 1.75±0.50 | 0.89±0.24 |
| 100 | 0.88±0.25 | 4.59±0.91* | 3.05±0.81* | 3.37±0.79* |
| 200 | 4.80±0.52* | 5.61±0.71* | 5.51±1.11* | 7.17±1.75* |
* p<0.05 compared with vehicle treatment (ANOVA, Dunnett’s t-test)
Mean increase in reaction time for tail withdrawal in vehicle treated mice was 0.43±0.61 sec and morphine (10 mg/kg, s.c) treated mice was16.80±0.94* sec
Discussion
Clinical use of common chemotherapeutic agents invariably causes painful neuropathy as a major morbidity. The disabling pain restricts the functional abilities of an individual and decreases the quality of life. The management of CIPN by anticonvulsants, antidepressants and opioids is only partially successful. Hence, novel compounds are being screened to provide adequate relief from CIPN and ensure the completion of chemotherapy. Flavone compounds possess analgesic, anti-inflammatory and antioxidant effects [16,17]. Based on these beneficial effects, a few flavones derivatives have been earlier investigated for their efficacy in CIPN induced by paclitaxel [18,19]. Flavone and its monohydroxy derivatives have been earlier reported to possess significant antinociceptive [10] and anti-inflammatory [5] effects in experimental animals. Hence, they were considered for the present investigation on CIPN induced by oxaliplatin and vincristine.
Administration of either vincristine or oxaliplatin to mice resulted in the manifestation of typical symptoms of neuropathy like, mechanical allodynia, cold allodynia and thermal hyperalgesia. An opioid analgesic morphine effectively blunted the expression of these symptoms. Treatment with flavone or its monohydroxy derivatives differentially modified these behavioural responses. The reduction of paw withdrawal response score in the von Frey’s hair aesthesiometer test revealed the ability of these compounds to attenuate the mechanical allodynia resulting from either vincristine or oxaliplatin administration [Table/Fig-2,3].
The mechanical allodynia resulting from vincristine was significantly reduced by 6-hydroxy flavone in a dose of 100 mg/kg [Table/Fig-2], whereas a higher dose of 200 mg/kg of flavone, 5-hydroxy flavone and 7-hydroxy flavone was required to produce a significant response. On the other hand, oxaliplatin induced mechanical allodynia was not effectively suppressed by 7-hydroxy flavone in any of the doses employed and a higher dose, (200 mg/kg) of flavone and 6-hydroxy flavone was required to produce a significant reduction [Table/Fig-3]. These results indicate that, mechanical allodynia developing after vincristine administration was more amenable to flavone derivatives than that induced by oxaliplatin.
Administration of vincristine or oxaliplatin also resulted in the development of cold allodynia in mice and this was effectively attenuated by treatment with different flavone derivatives [Table/Fig-4,5]. In vincristine treated animals, the paw withdrawal response score was significantly reduced by different flavone derivatives even in the minimal doses employed except 7-hydroxy flavone. However, higher doses of flavone and its derivatives were required to attenuate the cold allodynia developing after oxaliplatin administration [Table/Fig-5].
This observation raises a possibility that the intensity of cold allodynia due to oxaliplatin is more severe than due to vincristine and hence requires higher doses of the test compounds. In fact, cold allodynia has been described as the earliest symptom of peripheral neuropathy developing after oxaliplatin therapy and not commonly observed with other platinum compounds [20]. Thermal hyperalgesia is also a frequently encountered symptom of peripheral neuropathy due to cancer chemotherapeutic agents. In the present study, vincristine and oxaliplatin administration in mice resulted in thermal hyperalgesia which was effectively suppressed by morphine treatment.
Flavone treatment in doses of 100 and 200 mg/kg effectively attenuated thermal hyperalgesia due to vincristine administration [Table/Fig-6]. Whereas, 5-hydroxy flavone and 6-hydroxy flavone were effective only in a higher dose of 200 mg/kg and 7-hydroxy flavone was found to be ineffective to produce a significant response in all the doses tested. The pattern of response observed in oxaliplatin induced thermal hyperalgesia after treatment with flavone derivatives was different from that recorded for vincristine. All the three monohydroxy derivatives of flavone in doses of 100 and 200 mg/kg significantly attenuated thermal hyperalgesia due to oxaliplatin, while a higher dose of flavone (200 mg/kg) was required to produce a significant response [Table/Fig-7].
This observation indicates that, thermal hyperalgesia induced by oxaliplatin is more easily amenable to treatment with flavone and its monohydroxy derivatives. Perhaps, the neuropathic manifestation of thermal hyperalgesia due to vincristine was of more severe intensity and hence required higher doses of flavone derivatives for amelioration.
Perusal of the present results indicates the effectiveness of flavone and its monohydroxy derivatives in attenuation of the manifestations of CIPN induced by two important cancer chemotherapeutic drugs vincristine and oxaliplatin. However, some differences were observed in the effect of these flavone compounds in suppressing the symptoms of neuropathic behaviour developed after administration of these drugs. The differences in the nature of neurological damage caused by vincristine and oxaliplatin might also be responsible for the variations observed among the flavones.
Cold allodynia was effectively suppressed by flavone derivatives in the lower doses employed. Attenuation of thermal hyperalgesia and mechanical allodynia required higher doses of the test compounds. Moreover, mechanical allodynia due to oxaliplatin and thermal hyperalgesia due to vincristine were not reduced by 7-hydroxy flavone in the doses employed. These findings suggest that the neuropathic manifestations developing after vincristine and oxaliplatin are amenable to flavone and monohydroxy flavones in the following order;
Cold allodynia> Thermal hyperalgesia> Mechanical allodynia
The present data also reveals that, 5-hydroxy flavone and 6-hydroxy flavone are uniformly effective in suppressing the neuropathic symptoms of both vincristine and oxaliplatin. The efficacy of flavone can be considered next in order and 7-hydroxy flavone was ineffective against certain neuropathic manifestations as mentioned earlier. The overall efficacy of these compounds may be suggested as:
5-hydroxy flavone≥ 6-hydroxy flavone> 7-hydroxy flavone
A structure activity relationship was proposed earlier for the antinociceptive activity of flavone derivatives [10]. The flavone nucleus per se exhibited significant antinociceptive effect. Hydroxylation in the 5th or 6th position of flavone nucleus augmented the antinociceptive action of flavone. However, substitution of a hydroxyl group in the 7th position of flavone nucleus reduced the antinociceptive potency of flavone. Surprisingly, a similar pattern of response has also been recorded in the present study. The structure activity relationship proposed for the antinociceptive efficacy of flavone derivatives also holds good for their efficacy against CIPN.
Even though the precise mechanism of action of these compounds in CIPN cannot be defined, evidences are available from the literature to suggest possible modes of action of flavone derivatives. An opiate like mechanism was found to be involved in the anti-nociceptive action of flavone, 5-hydroxy flavone and 7-hydroxy flavone [10]. This may be suggested as one of the mechanisms for the results observed in the present study.
In a recent study, flavonol (3-hydroxy flavone) and its dimethoxy derivatives have been found to be effective in attenuating paclitaxel induced neuropathy in mice [18]. Inhibition of pro-inflammatory cytokines like TNF-α, IL1-β and free radicals has been suggested as a possible mechanism of action of these flavonols. In fact, the test compounds employed in the present study have been earlier reported to possess significant anti-inflammatory effect which may contribute to the beneficial effect in CIPN [5]. An analysis on the effect of monohydroxy flavones on inflammatory mediators may validate the present findings further.
An alteration in the activity of neuronal ion channels like sodium and calcium has been implicated in the development of CIPN [12]. In particular, agents that modify calcium channel activity like ethosuximide have been found to be effective in reversing paclitaxel and vincristine induced neuropathy [14]. Gabapentin, which has been shown to bind to α2δ sub-unit of calcium channel is an established drug in the treatment of peripheral neuropathy [2]. A previous report indicates that the analgesic effect of flavone and its hydroxy derivatives was antagonized by i.v administration of calcium and a calcium channel blocker nifedipine potentiated this response [21]. This report suggests a possible role of flavones in modulating calcium channel activity and indicates a potential mechanism by which CIPN may be attenuated by flavones.
Mitochondrial generated reactive oxygen species contribute to CIPN by activating proinflammatory pathways [22] and sensitisation of TRPA1 channels with enhanced thermal sensitivity [23]. 3-hydroxy flavone and 7-hydroxy flavone offered protection against nicotine-induced cytotoxicity in cultured renal proximal tubular cells by inhibition of nicotine-induced reactive oxygen species [24].
The antioxidant activity of flavonol (3-hydroxy flavone) and its methoxy derivatives has been proposed as a possible mechanism for protection against CIPN induced by paclitaxel in mice [25]. The flavone derivatives employed in the present study may also exert similar antioxidant profile to offer protection against CIPN resulting from oxaliplatin or vincristine administration. However, further experiments may be needed to confirm this proposal.
Limitation(s)
The present investigation was limited to screening a few monohydroxy flavones for their effect on CIPN in a single animal species. Confirmation of these findings in another animal species like rat which is also widely used in neuropathy studies may further strengthen the present results. Further, a detailed structure activity relationship among flavone compounds may be envisaged by investigating polyhydroxy flavones and other derivatives of flavone to identify the most effective candidates among them to treat CIPN.
Conclusion(s)
The present study has identified the effectiveness of structurally related monohydroxy flavone derivatives in alleviating the manifestations of CIPN induced by two important cancer chemotherapeutic drugs, vincristine and oxaliplatin. The results further underscore the importance of flavonoids as a potential source of drugs effective in the management of CIPN.